An independent endocytic pathway stimulates different monocyte subsets by the a2 N-terminus domain of vacuolar-ATPase
- PMID: 23483532
- PMCID: PMC3583941
- DOI: 10.4161/onci.22978
An independent endocytic pathway stimulates different monocyte subsets by the a2 N-terminus domain of vacuolar-ATPase
Abstract
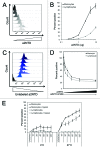
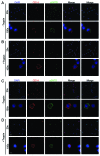
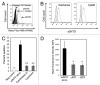
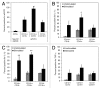
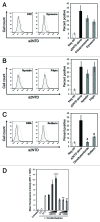
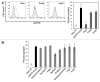
The vacuolar ATPase (V-ATPase) plays an important role in tumor progression and metastases. A novel peptide from the a2 isoform of V-ATPase called a2NTD has been shown to exert an immunoregulatory role in the tumor microenvironment by controlling the maturation of monocytes toward a tumor-associated macrophage phenotype. Our data indicate that a2NTD binds to the surface of monocytes. a2NTD was preferentially endocytosed by pro-inflammatory monocytes bearing a CD14++CD16+ phenotype, which is associated with the monocyte-to-macrophage maturation process. Both a2NTD binding and internalization led to production of the pro-inflammatory cytokines interleukin (IL)-1α and IL-1β by CD14++CD16- (classical) and CD14++CD16+ (intermediate) monocytes. a2NTD was internalized via a macropinocytosis mechanism utilizing scavenger receptors. However, the inhibition of a2NTD endocytosis did not reduce cytokine production by monocytes. This points to the existence of two receptors that respond to a2NTD: scavengers receptors that mediate cellular uptake and an hitherto unidentified receptor stimulating the production of inflammatory cytokines. Both of these monocyte receptors may be important in generating the localized inflammation that is often required to promote tumor growth and hence may constitute novel targets for the development of anticancer drugs.
Keywords: CD14; CD16; IL-1; cancer-related inflammation; pro-inflammatory monocytes; tumor associated macrophages; vacuolar ATPase.
Figures
Similar articles
-
Novel role for the N-terminus domain of the a2 isoform of vacuolar ATPase in interleukin-1beta production.Hum Immunol. 2007 Jun;68(6):469-77. doi: 10.1016/j.humimm.2007.02.010. Epub 2007 Mar 26. Hum Immunol. 2007. PMID: 17509446
-
The N-terminus domain of the a2 isoform of vacuolar ATPase can regulate interleukin-1beta production from mononuclear cells in co-culture with JEG-3 choriocarcinoma cells.Am J Reprod Immunol. 2007 Mar;57(3):201-9. doi: 10.1111/j.1600-0897.2006.00463.x. Am J Reprod Immunol. 2007. PMID: 17295899
-
Tumor-associated vacuolar ATPase subunit promotes tumorigenic characteristics in macrophages.Oncogene. 2014 Dec 4;33(49):5649-54. doi: 10.1038/onc.2013.532. Epub 2013 Dec 23. Oncogene. 2014. PMID: 24362525
-
Tumor-associated a2 vacuolar ATPase acts as a key mediator of cancer-related inflammation by inducing pro-tumorigenic properties in monocytes.J Immunol. 2011 Feb 1;186(3):1781-9. doi: 10.4049/jimmunol.1002998. Epub 2010 Dec 22. J Immunol. 2011. PMID: 21178005
-
Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis.Int Immunopharmacol. 2018 Dec;65:348-359. doi: 10.1016/j.intimp.2018.10.016. Epub 2018 Oct 23. Int Immunopharmacol. 2018. PMID: 30366278 Review.
Cited by
-
CD44‑hyaluronan axis plays a role in the interactions between colon cancer‑derived extracellular vesicles and human monocytes.Oncol Lett. 2023 Aug 7;26(3):413. doi: 10.3892/ol.2023.13999. eCollection 2023 Sep. Oncol Lett. 2023. PMID: 37600336 Free PMC article.
-
Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor.J Immunol. 2013 Dec 1;191(11):5702-13. doi: 10.4049/jimmunol.1301604. Epub 2013 Oct 25. J Immunol. 2013. PMID: 24163412 Free PMC article.
-
Determination of cell uptake pathways for tumor inhibitor lysyl oxidase propeptide.Mol Oncol. 2016 Jan;10(1):1-23. doi: 10.1016/j.molonc.2015.07.005. Epub 2015 Aug 6. Mol Oncol. 2016. PMID: 26297052 Free PMC article.
-
The Vacuolar ATPase a2-subunit regulates Notch signaling in triple-negative breast cancer cells.Oncotarget. 2015 Oct 27;6(33):34206-20. doi: 10.18632/oncotarget.5275. Oncotarget. 2015. PMID: 26418877 Free PMC article.
-
Inhibition of vacuolar ATPase subunit in tumor cells delays tumor growth by decreasing the essential macrophage population in the tumor microenvironment.Oncogene. 2016 Feb 25;35(8):1058-65. doi: 10.1038/onc.2015.159. Epub 2015 May 11. Oncogene. 2016. PMID: 25961933
References
-
- Martinez-Zaguilan R, Lynch RM, Martinez GM, Gillies RJ. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol. 1993;265:C1015–29. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
