Oxidative stress in hypertension: role of the kidney
- PMID: 23472618
- PMCID: PMC3880923
- DOI: 10.1089/ars.2013.5259
Oxidative stress in hypertension: role of the kidney
Abstract
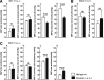
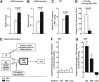
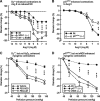
Significance: Renal oxidative stress can be a cause, a consequence, or more often a potentiating factor for hypertension. Increased reactive oxygen species (ROS) in the kidney have been reported in multiple models of hypertension and related to renal vasoconstriction and alterations of renal function. Nicotinamide adenine dinucleotide phosphate oxidase is the central source of ROS in the hypertensive kidney, but a defective antioxidant system also can contribute.
Recent advances: Superoxide has been identified as the principal ROS implicated for vascular and tubular dysfunction, but hydrogen peroxide (H2O2) has been implicated in diminishing preglomerular vascular reactivity, and promoting medullary blood flow and pressure natriuresis in hypertensive animals.
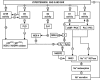
Critical issues and future directions: Increased renal ROS have been implicated in renal vasoconstriction, renin release, activation of renal afferent nerves, augmented contraction, and myogenic responses of afferent arterioles, enhanced tubuloglomerular feedback, dysfunction of glomerular cells, and proteinuria. Inhibition of ROS with antioxidants, superoxide dismutase mimetics, or blockers of the renin-angiotensin-aldosterone system or genetic deletion of one of the components of the signaling cascade often attenuates or delays the onset of hypertension and preserves the renal structure and function. Novel approaches are required to dampen the renal oxidative stress pathways to reduced O2(-•) rather than H2O2 selectivity and/or to enhance the endogenous antioxidant pathways to susceptible subjects to prevent the development and renal-damaging effects of hypertension.
Figures

Similar articles
-
Reactive oxygen species in renal vascular function.Acta Physiol (Oxf). 2020 Aug;229(4):e13477. doi: 10.1111/apha.13477. Epub 2020 May 8. Acta Physiol (Oxf). 2020. PMID: 32311827 Review.
-
Redox regulation of the afferent arteriole and tubuloglomerular feedback.Acta Physiol Scand. 2003 Nov;179(3):217-23. doi: 10.1046/j.0001-6772.2003.01205.x. Acta Physiol Scand. 2003. PMID: 14616237 Review.
-
Oxidant Mechanisms in Renal Injury and Disease.Antioxid Redox Signal. 2016 Jul 20;25(3):119-46. doi: 10.1089/ars.2016.6665. Epub 2016 Apr 26. Antioxid Redox Signal. 2016. PMID: 26906267 Free PMC article. Review.
-
Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research.Antioxid Redox Signal. 2014 Jan 1;20(1):164-82. doi: 10.1089/ars.2013.5302. Epub 2013 Jun 6. Antioxid Redox Signal. 2014. PMID: 23600794 Free PMC article. Review.
-
Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance?Hypertension. 2004 Sep;44(3):248-52. doi: 10.1161/01.HYP.0000138070.47616.9d. Epub 2004 Jul 19. Hypertension. 2004. PMID: 15262903 Review.
Cited by
-
Remodeling of Afferent Arterioles From Mice With Oxidative Stress Does Not Account for Increased Contractility but Does Limit Excessive Wall Stress.Hypertension. 2015 Sep;66(3):550-6. doi: 10.1161/HYPERTENSIONAHA.115.05631. Epub 2015 Jun 22. Hypertension. 2015. PMID: 26101341 Free PMC article.
-
Thromboxane prostanoid receptors enhance contractions, endothelin-1, and oxidative stress in microvessels from mice with chronic kidney disease.Hypertension. 2015 May;65(5):1055-63. doi: 10.1161/HYPERTENSIONAHA.115.05244. Epub 2015 Mar 2. Hypertension. 2015. PMID: 25733239 Free PMC article.
-
Coordinated Contribution of NADPH Oxidase- and Mitochondria-Derived Reactive Oxygen Species in Metabolic Syndrome and Its Implication in Renal Dysfunction.Front Pharmacol. 2021 May 4;12:670076. doi: 10.3389/fphar.2021.670076. eCollection 2021. Front Pharmacol. 2021. PMID: 34017260 Free PMC article. Review.
-
The potential role of complement alternative pathway activation in hypertensive renal damage.Exp Biol Med (Maywood). 2022 May;247(9):797-804. doi: 10.1177/15353702221091986. Epub 2022 Apr 27. Exp Biol Med (Maywood). 2022. PMID: 35473318 Free PMC article.
-
Hydrogen peroxide derived from NADPH oxidase 4- and 2 contributes to the endothelium-dependent vasodilatation of intrarenal arteries.Redox Biol. 2018 Oct;19:92-104. doi: 10.1016/j.redox.2018.08.004. Epub 2018 Aug 7. Redox Biol. 2018. PMID: 30125808 Free PMC article.
References
-
- Adler S. and Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol 287: F907–F913, 2004 - PubMed
-
- Ahmeda AF. and Johns EJ. The regulation of blood perfusion in the renal cortex and medulla by reactive oxygen species and nitric oxide in the anaesthetised rat. Acta Physiol (Oxf) 204: 443–450, 2012 - PubMed
-
- Aldred KL, Harris PJ, and Eitle E. Increased proximal tubule NHE-3 and H+-ATPase activities in spontaneously hypertensive rats. J Hypertens 18: 623–628, 2000 - PubMed
-
- Alexander MY, Brosnan MJ, Hamilton CA, Fennell JP, Beattie EC, Jardine E, Heistad DD, and Dominiczak AF. Gene transfer of endothelial nitric oxide synthase but not Cu/Zn superoxide dismutase restores nitric oxide availability in the SHRSP. Cardiovasc Res 47: 609–617, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

