Sustained activation of sphingomyelin synthase by 2-hydroxyoleic acid induces sphingolipidosis in tumor cells
- PMID: 23471028
- PMCID: PMC3653406
- DOI: 10.1194/jlr.M036749
Sustained activation of sphingomyelin synthase by 2-hydroxyoleic acid induces sphingolipidosis in tumor cells
Abstract
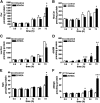
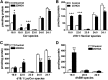
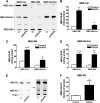
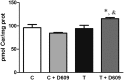
The mechanism of action of 2-hydroxyoleic acid (2OHOA), a potent antitumor drug, involves the rapid and specific activation of sphingomyelin synthase (SMS), leading to a 4-fold increase in SM mass in tumor cells. In the present study, we investigated the source of the ceramides required to sustain this dramatic increase in SM. Through radioactive and fluorescent labeling, we demonstrated that sphingolipid metabolism was altered by a 24 h exposure to 2OHOA, and we observed a consistent increase in the number of lysosomes and the presence of unidentified storage materials in treated cells. Mass spectroscopy revealed that different sphingolipid classes accumulated in human glioma U118 cells after exposure to 2OHOA, demonstrating a specific effect on C16-, C20-, and C22-containing sphingolipids. Based on these findings, we propose that the demand for ceramides required to sustain the SMS activation (ca. 200-fold higher than the basal level) profoundly modifies both sphingolipid and phospholipid metabolism. As the treatment is prolonged, tumor cells fail to adequately metabolize sphingolipids, leading to a situation resembling sphingolipidosis, whereby cell viability is compromised.
Figures

Similar articles
-
Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy.Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19569-74. doi: 10.1073/pnas.1115484108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106271 Free PMC article.
-
Differential effect of 2-hydroxyoleic acid enantiomers on protein (sphingomyelin synthase) and lipid (membrane) targets.Biochim Biophys Acta. 2014 Jun;1838(6):1628-37. doi: 10.1016/j.bbamem.2013.12.023. Epub 2014 Jan 9. Biochim Biophys Acta. 2014. PMID: 24412218
-
2-Hydroxy-oleic acid does not activate sphingomyelin synthase activity.J Biol Chem. 2018 Nov 23;293(47):18328-18336. doi: 10.1074/jbc.RA118.005904. Epub 2018 Oct 10. J Biol Chem. 2018. PMID: 30305392 Free PMC article.
-
[Ceramide-centered sphingolipid metabolism].Seikagaku. 2011 Jun;83(6):495-505. Seikagaku. 2011. PMID: 21800680 Review. Japanese. No abstract available.
-
Sphingolipids and neuronal degeneration in lysosomal storage disorders.J Neurochem. 2019 Mar;148(5):600-611. doi: 10.1111/jnc.14540. Epub 2018 Aug 16. J Neurochem. 2019. PMID: 29959861 Review.
Cited by
-
2'-Hydroxy ceramide in membrane homeostasis and cell signaling.Adv Biol Regul. 2014 Jan;54:223-30. doi: 10.1016/j.jbior.2013.09.012. Epub 2013 Oct 8. Adv Biol Regul. 2014. PMID: 24139861 Free PMC article. Review.
-
A Phase 1/2A trial of idroxioleic acid: first-in-class sphingolipid regulator and glioma cell autophagy inducer with antitumor activity in refractory glioma.Br J Cancer. 2023 Sep;129(5):811-818. doi: 10.1038/s41416-023-02356-1. Epub 2023 Jul 24. Br J Cancer. 2023. PMID: 37488446 Free PMC article. Clinical Trial.
-
Sphingomyelin Synthase 1 Regulates Neuro-2a Cell Proliferation and Cell Cycle Progression Through Modulation of p27 Expression and Akt Signaling.Mol Neurobiol. 2015;51(3):1530-41. doi: 10.1007/s12035-014-8829-z. Epub 2014 Aug 2. Mol Neurobiol. 2015. PMID: 25084761 Free PMC article.
-
Investigating the impact of 2-OHOA-embedded liposomes on biophysical properties of cancer cell membranes via Laurdan two-photon microscopy imaging.Sci Rep. 2024 Jul 9;14(1):15831. doi: 10.1038/s41598-024-65812-9. Sci Rep. 2024. PMID: 38982188 Free PMC article.
-
Targeting the Sphingolipid Rheostat in Gliomas.Int J Mol Sci. 2022 Aug 17;23(16):9255. doi: 10.3390/ijms23169255. Int J Mol Sci. 2022. PMID: 36012521 Free PMC article. Review.
References
-
- Martínez J., Gutiérrez A., Casas J., Lladó V., López-Bellán A., Besalduch J., Dopazo A., Escribá P. V. 2005. The repression of E2F-1 is critical for the activity of Minerval against cancer. J. Pharmacol. Exp. Ther. 315: 466–474 - PubMed
-
- Terés S., Lladó V., Higuera M., Barceló-Coblijn G., Martin M. L., Noguera-Salvà M. A., Marcilla-Etxenike A., García-Verdugo J. M., Soriano-Navarro M., Saus C., et al. 2012. 2-Hydroxyoleate, a nontoxic membrane binding anticancer drug, induces glioma cell differentiation and autophagy. Proc. Natl. Acad. Sci. USA. 109: 8489–8494 - PMC - PubMed
-
- Martínez J., Vögler O., Casas J., Barceló F., Alemany R., Prades J., Nagy T., Baamonde C., Kasprzyk P. G., Terés S., et al. 2005. Membrane structure modulation, protein kinase C alpha activation, and anticancer activity of minerval. Mol. Pharmacol. 67: 531–540 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

