Cysteine protease enhances plant-mediated bollworm RNA interference
- PMID: 23460027
- PMCID: PMC3755213
- DOI: 10.1007/s11103-013-0030-7
Cysteine protease enhances plant-mediated bollworm RNA interference
Abstract
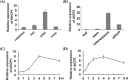
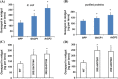
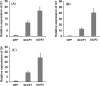
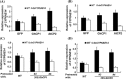
Oral ingestion of plant-expressed double stranded RNA (dsRNA) triggers target gene suppression in insect. An important step of this process is the transmission of dsRNA from plant to midgut cells. Insect peritrophic matrix (PM) presents a barrier that prevents large molecules from entering midgut cells. Here, we show that uptake of plant cysteine proteases, such as GhCP1 from cotton (Gossypium hirsutum) and AtCP2 from Arabidopsis, by cotton bollworm (Helicoverpa armigera) larvae resulted in attenuating the PM. When GhCP1 or AtCP2 pre-fed larvae were transferred to gossypol-containing diet, the bollworm accumulated higher content of gossypol in midgut. Larvae previously ingested GhCP1 or AtCP2 were more susceptible to infection by Dendrolimus punctatus cytoplasmic polyhedrosis virus (DpCPV), a dsRNA virus. Furthermore, the pre-fed larvae exhibited enhanced RNAi effects after ingestion of the dsRNA-expressing plant. The bollworm P450 gene CYP6AE14 is involved in the larval tolerance to gossypol; cotton plants producing dsRNA of CYP6AE14 (dsCYP6AE14) were more resistant to bollworm feeding (Mao et al. in Transgenic Res 20:665-673, 2011). We found that cotton plants harboring both 35S:dsCYP6AE14 and 35S:GhCP1 were better protected from bollworm than either of the single-transgene lines. Our results demonstrate that plant cysteine proteases, which have the activity of increasing PM permeability, can be used to improve the plant-mediated RNAi against herbivorous insects.
Figures


Similar articles
-
Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms.Transgenic Res. 2011 Jun;20(3):665-73. doi: 10.1007/s11248-010-9450-1. Epub 2010 Oct 17. Transgenic Res. 2011. PMID: 20953975 Free PMC article.
-
Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol.Nat Biotechnol. 2007 Nov;25(11):1307-13. doi: 10.1038/nbt1352. Epub 2007 Nov 4. Nat Biotechnol. 2007. PMID: 17982444
-
Gossypol-enhanced P450 gene pool contributes to cotton bollworm tolerance to a pyrethroid insecticide.Mol Ecol. 2012 Sep;21(17):4371-85. doi: 10.1111/j.1365-294X.2012.05548.x. Epub 2012 Apr 20. Mol Ecol. 2012. PMID: 22515600
-
Diet-delivered RNAi in Helicoverpa armigera--Progresses and challenges.J Insect Physiol. 2016 Feb;85:86-93. doi: 10.1016/j.jinsphys.2015.11.005. Epub 2015 Nov 5. J Insect Physiol. 2016. PMID: 26549127 Review.
-
Next-Generation Insect-Resistant Plants: RNAi-Mediated Crop Protection.Trends Biotechnol. 2017 Sep;35(9):871-882. doi: 10.1016/j.tibtech.2017.04.009. Epub 2017 Jul 19. Trends Biotechnol. 2017. PMID: 28822479 Review.
Cited by
-
Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities.Ann Bot. 2015 Jun;115(7):1015-51. doi: 10.1093/aob/mcv054. Ann Bot. 2015. PMID: 26019168 Free PMC article. Review.
-
Krüppel-homologue 1 regulates the development of Tuta absoluta and its cascade regulation pattern in the juvenile hormone signalling pathway.Open Biol. 2023 May;13(5):220372. doi: 10.1098/rsob.220372. Epub 2023 May 31. Open Biol. 2023. PMID: 37253420 Free PMC article.
-
Selection of reference genes for RT-qPCR analysis in a predatory biological control agent, Coleomegilla maculata (Coleoptera: Coccinellidae).Sci Rep. 2015 Dec 10;5:18201. doi: 10.1038/srep18201. Sci Rep. 2015. PMID: 26656102 Free PMC article.
-
Assessment of Potential Risks of Dietary RNAi to a Soil Micro-arthropod, Sinella curviseta Brook (Collembola: Entomobryidae).Front Plant Sci. 2016 Jul 15;7:1028. doi: 10.3389/fpls.2016.01028. eCollection 2016. Front Plant Sci. 2016. PMID: 27471512 Free PMC article.
-
Application progress of plant-mediated RNAi in pest control.Front Bioeng Biotechnol. 2022 Aug 8;10:963026. doi: 10.3389/fbioe.2022.963026. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36003536 Free PMC article. Review.
References
-
- Benbouza H, Baudoin JP, Mergeai G. Improvement of the genomic DNA extraction method with CTAB for cotton leaves. Biotechnologie Agronomie Societe et Environ. 2006;10:73–76.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

