Identification of the amino acid residues and domains in the cysteine-rich protein of Chinese wheat mosaic virus that are important for RNA silencing suppression and subcellular localization
- PMID: 23458485
- PMCID: PMC6638639
- DOI: 10.1111/mpp.12002
Identification of the amino acid residues and domains in the cysteine-rich protein of Chinese wheat mosaic virus that are important for RNA silencing suppression and subcellular localization
Abstract
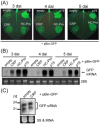
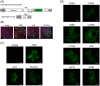
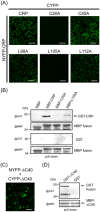
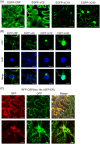
Cysteine-rich proteins (CRPs) encoded by some plant viruses in diverse genera function as RNA silencing suppressors. Within the N-terminal portion of CRPs encoded by furoviruses, there are six conserved cysteine residues and a Cys-Gly-X-X-His motif (Cys, cysteine; Gly, glycine; His, histidine; X, any amino acid residue) with unknown function. The central domains contain coiled-coil heptad amino acid repeats that usually mediate protein dimerization. Here, we present evidence that the conserved cysteine residues and Cys-Gly-X-X-His motif in the CRP of Chinese wheat mosaic virus (CWMV) are critical for protein stability and silencing suppression activity. Mutation of a leucine residue in the third coiled-coil heptad impaired CWMV CRP activity for suppression of local silencing, but not for the promotion of cell-to-cell movement of Potato virus X (PVX). In planta and in vitro analysis of wild-type and mutant proteins indicated that the ability of the CRP to self-interact was correlated with its suppression activity. Deletion of up to 40 amino acids at the C-terminus did not abolish suppression activity, but disrupted the association of CRP with endoplasmic reticulum (ER), and reduced its activity in the enhancement of PVX symptom severity. Interestingly, a short region in the C-terminal domain, predicted to form an amphipathic α-helical structure, was responsible for the association of CWMV CRP with ER. Overall, our results demonstrate that the N-terminal and central regions are the functional domains for suppression activity, whereas the C-terminal region primarily functions to target CWMV CRP to the ER.
© 2012 THE AUTHORS. MOLECULAR PLANT PATHOLOGY © 2012 BSPP AND BLACKWELL PUBLISHING LTD.
Figures


Similar articles
-
Endoplasmic reticulum export and vesicle formation of the movement protein of Chinese wheat mosaic virus are regulated by two transmembrane domains and depend on the secretory pathway.Virology. 2013 Jan 20;435(2):493-503. doi: 10.1016/j.virol.2012.10.024. Epub 2012 Nov 6. Virology. 2013. PMID: 23137810
-
Complete sequence and genome properties of Chinese wheat mosaic virus, a new furovirus from China.J Gen Virol. 1999 May;80 ( Pt 5):1141-1145. doi: 10.1099/0022-1317-80-5-1141. J Gen Virol. 1999. PMID: 10355760
-
N-terminal region of cysteine-rich protein (CRP) in carlaviruses is involved in the determination of symptom types.Mol Plant Pathol. 2018 Jan;19(1):180-190. doi: 10.1111/mpp.12513. Epub 2017 Jan 25. Mol Plant Pathol. 2018. PMID: 27868376 Free PMC article.
-
The CUG-initiated larger form coat protein of Chinese wheat mosaic virus binds to the cysteine-rich RNA silencing suppressor.Virus Res. 2013 Oct;177(1):66-74. doi: 10.1016/j.virusres.2013.07.013. Epub 2013 Jul 30. Virus Res. 2013. PMID: 23911633
-
Soilborne wheat mosaic virus (SBWMV) 19K protein belongs to a class of cysteine rich proteins that suppress RNA silencing.Virol J. 2005 Mar 1;2:18. doi: 10.1186/1743-422X-2-18. Virol J. 2005. PMID: 15740624 Free PMC article.
Cited by
-
Chinese Wheat Mosaic Virus-Induced Gene Silencing in Monocots and Dicots at Low Temperature.Front Plant Sci. 2018 Nov 14;9:1627. doi: 10.3389/fpls.2018.01627. eCollection 2018. Front Plant Sci. 2018. PMID: 30487803 Free PMC article.
-
Zn2+-dependent association of cysteine-rich protein with virion orchestrates morphogenesis of rod-shaped viruses.PLoS Pathog. 2024 Jun 17;20(6):e1012311. doi: 10.1371/journal.ppat.1012311. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38885273 Free PMC article.
-
Large-scale phosphoproteome analysis in wheat seedling leaves provides evidence for extensive phosphorylation of regulatory proteins during CWMV infection.BMC Plant Biol. 2023 Nov 2;23(1):532. doi: 10.1186/s12870-023-04559-3. BMC Plant Biol. 2023. PMID: 37914991 Free PMC article.
-
Coat protein of rice stripe virus enhances autophagy activity through interaction with cytosolic glyceraldehyde-3-phosphate dehydrogenases, a negative regulator of plant autophagy.Stress Biol. 2023 Mar 23;3(1):3. doi: 10.1007/s44154-023-00084-3. Stress Biol. 2023. PMID: 37676568 Free PMC article.
-
A critical domain of Sweet potato chlorotic fleck virus nucleotide-binding protein (NaBp) for RNA silencing suppression, nuclear localization and viral pathogenesis.Mol Plant Pathol. 2015 May;16(4):365-75. doi: 10.1111/mpp.12186. Epub 2014 Sep 24. Mol Plant Pathol. 2015. PMID: 25138489 Free PMC article.
References
-
- Adams, M.J. , Heinze, C. , Jackson, A.O. , Kreuze, J. , Macfarlane, S.A. and Torrance, L. (2011) Family Virgaviridae In: Virus Taxonomy, Ninth Report of the International Committee on Taxonomy of Viruses (King A.M.Q., Adams M.J., Carstens E.B. and Lefkowitz E.J., eds), pp. 1139–1162. London: Elsevier Academic Press.
-
- Andika, I.B. , Kondo, H. and Tamada, T. (2005) Evidence that RNA silencing‐mediated resistance to Beet necrotic yellow vein virus is less effective in roots than in leaves. Mol. Plant–Microbe Interact. 18, 194–204. - PubMed
-
- Andika, I.B. , Kondo, H. , Nishiguchi, M. and Tamada, T. (2012) The cysteine‐rich proteins of beet necrotic yellow vein virus and tobacco rattle virus contribute to efficient suppression of silencing in roots. J. Gen. Virol. 93, 1841–1850. - PubMed
-
- Baulcombe, D. (2005) RNA silencing. Trends Biochem. Sci. 30, 290–293. - PubMed
-
- Bayne, E.H. , Rakitina, D.V. , Morozov, S.Y. and Baulcombe, D.C. (2005) Cell‐to‐cell movement of Potato Potexvirus X is dependent on suppression of RNA silencing. Plant J. 44, 471–482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

