Peptide YY3-36 and 5-hydroxytryptamine mediate emesis induction by trichothecene deoxynivalenol (vomitoxin)
- PMID: 23457120
- PMCID: PMC3627556
- DOI: 10.1093/toxsci/kft033
Peptide YY3-36 and 5-hydroxytryptamine mediate emesis induction by trichothecene deoxynivalenol (vomitoxin)
Abstract
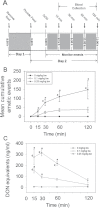
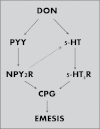
Deoxynivalenol (DON, vomitoxin), a trichothecene mycotoxin produced by Fusarium sp. that frequently occurs in cereal grains, has been associated with human and animal food poisoning. Although a common hallmark of DON-induced toxicity is the rapid onset of emesis, the mechanisms for this adverse effect are not fully understood. Recently, our laboratory has demonstrated that the mink (Neovison vison) is a suitable small animal model for investigating trichothecene-induced emesis. The goal of this study was to use this model to determine the roles of two gut satiety hormones, peptide YY3-36 (PYY3-36) and cholecystokinin (CCK), and the neurotransmitter 5-hydroxytryptamine (5-HT) in DON-induced emesis. Following ip exposure to DON at 0.1 and 0.25mg/kg bw, emesis induction ensued within 15-30min and then persisted up to 120min. Plasma DON measurement revealed that this emesis period correlated with the rapid distribution and clearance of the toxin. Significant elevations in both plasma PYY3-36 (30-60min) and 5-HT (60min) but not CCK were observed during emesis. Pretreatment with the neuropeptide Y2 receptor antagonist JNJ-31020028 attenuated DON- and PYY-induced emesis, whereas the CCK1 receptor antagonist devezapide did not alter DON's emetic effects. The 5-HT3 receptor antagonist granisetron completely suppressed induction of vomiting by DON and the 5-HT inducer cisplatin. Granisetron pretreatment also partially blocked PYY3-36-induced emesis, suggesting a potential upstream role for this gut satiety hormone in 5-HT release. Taken together, the results suggest that both PYY3-36 and 5-HT play contributory roles in DON-induced emesis.
Figures



Similar articles
-
Emetic responses to T-2 toxin, HT-2 toxin and emetine correspond to plasma elevations of peptide YY3-36 and 5-hydroxytryptamine.Arch Toxicol. 2016 Apr;90(4):997-1007. doi: 10.1007/s00204-015-1508-7. Epub 2015 Apr 9. Arch Toxicol. 2016. PMID: 25855062 Free PMC article.
-
Type A Trichothecene Diacetoxyscirpenol-Induced Emesis Corresponds to Secretion of Peptide YY and Serotonin in Mink.Toxins (Basel). 2020 Jun 25;12(6):419. doi: 10.3390/toxins12060419. Toxins (Basel). 2020. PMID: 32630472 Free PMC article.
-
Calcium-Sensing Receptor and Transient Receptor Ankyrin-1 Mediate Emesis Induction by Deoxynivalenol (Vomitoxin).Toxicol Sci. 2017 Jan;155(1):32-42. doi: 10.1093/toxsci/kfw191. Epub 2016 Sep 25. Toxicol Sci. 2017. PMID: 27667315 Free PMC article.
-
Current and prospective sights in mechanism of deoxynivalenol-induced emesis for future scientific study and clinical treatment.J Appl Toxicol. 2017 Jul;37(7):784-791. doi: 10.1002/jat.3433. Epub 2017 Jan 31. J Appl Toxicol. 2017. PMID: 28138998 Review.
-
[Serotonin and anticancer drug-induced emesis].Yakugaku Zasshi. 2004 Aug;124(8):491-507. doi: 10.1248/yakushi.124.491. Yakugaku Zasshi. 2004. PMID: 15297719 Review. Japanese.
Cited by
-
Vitamin 25(OH)D3, E, and C Supplementation Impact the Inflammatory and Antioxidant Responses in Piglets Fed a Deoxynivalenol-Contaminated Diet and Challenged with Lipopolysaccharides.Toxins (Basel). 2024 Jun 28;16(7):297. doi: 10.3390/toxins16070297. Toxins (Basel). 2024. PMID: 39057937 Free PMC article.
-
Emetic responses to T-2 toxin, HT-2 toxin and emetine correspond to plasma elevations of peptide YY3-36 and 5-hydroxytryptamine.Arch Toxicol. 2016 Apr;90(4):997-1007. doi: 10.1007/s00204-015-1508-7. Epub 2015 Apr 9. Arch Toxicol. 2016. PMID: 25855062 Free PMC article.
-
Modeling the emetic potencies of food-borne trichothecenes by benchmark dose methodology.Food Chem Toxicol. 2016 Aug;94:178-85. doi: 10.1016/j.fct.2016.06.009. Epub 2016 Jun 10. Food Chem Toxicol. 2016. PMID: 27292944 Free PMC article.
-
Mechanisms of Nausea and Vomiting: Current Knowledge and Recent Advances in Intracellular Emetic Signaling Systems.Int J Mol Sci. 2021 May 28;22(11):5797. doi: 10.3390/ijms22115797. Int J Mol Sci. 2021. PMID: 34071460 Free PMC article. Review.
-
Investigation of the Efficacy of a Postbiotic Yeast Cell Wall-Based Blend on Newly-Weaned Pigs under a Dietary Challenge of Multiple Mycotoxins with Emphasis on Deoxynivalenol.Toxins (Basel). 2020 Aug 6;12(8):504. doi: 10.3390/toxins12080504. Toxins (Basel). 2020. PMID: 32781569 Free PMC article.
References
-
- Abbott C. R., Monteiro M., Small C. J., Sajedi A., Smith K. L., Parkinson J. R., Ghatei M. A., Bloom S. R. (2005). The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 1044, 127–131 - PubMed
-
- Andrews P. L., Davis C. J., Bingham S., Davidson H. I., Hawthorn J., Maskell L. (1990). The abdominal visceral innervation and the emetic reflex: Pathways, pharmacology, and plasticity. Can. J. Physiol. Pharmacol. 68, 325–345 - PubMed
-
- Andrews P. L., Hawthorn J. (1988). The neurophysiology of vomiting. Baillieres Clin. Gastroenterol. 2, 141–168 - PubMed
-
- Anonymous (1999). Outbreaks of gastrointestinal illness of unknown etiology associated with eating burritos—United States, October 1997-October 1998. MMWR Morb. Mortal. Wkly. Rep. 48, 210–213 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

