The hepatic cannabinoid 1 receptor as a modulator of hepatic energy state and food intake
- PMID: 23452341
- PMCID: PMC3895344
- DOI: 10.1111/bcp.12102
The hepatic cannabinoid 1 receptor as a modulator of hepatic energy state and food intake
Abstract
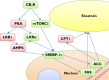
The cannabinoid 1 receptor (CB1R) has a well-established role in appetite regulation. Central CB1R antagonists, notably rimonabant, induced weight loss and improved the metabolic profile in obese individuals, but were discontinued due to psychiatric side-effects. The CB1R is also expressed peripherally, where its effects include promotion of liver fat accumulation, which consumes ATP. Type 2 diabetes in obese subjects is linked to excess liver fat, whilst there is a negative correlation between hepatic ATP content and insulin resistance. A decreased hepatic ATP/AMP ratio increases food intake by signals via the vagus nerve to the brain. The hepatic cannabinoid system is highly upregulated in obesity, and the effects of hepatic CB1R activation include increased activity of lipogenic and gluconeogenic transcription factors. Thus, blockade of hepatic CB1Rs could contribute significantly to the weight-reducing and insulin-sensitizing effects of CB1R antagonists. Additionally, upregulation of the hepatic CB1R may contribute to chronic liver inflammation, fibrosis and cirrhosis from causes including obesity, alcoholism and viral hepatitis. Peripheral CB1R antagonists induce weight loss and metabolic improvements in obese rodents; however, as there is evidence that hepatic CB1Rs are predominately intracellular, due to high intrinsic clearance, many drugs may not effectively block these receptors and therefore have limited efficacy. Hepatoselective CB1R antagonists may be effective at reducing hepatic steatosis, insulin resistance and bodyweight in obese, diabetic patients, with far fewer side-effects than first-generation CB1R antagonists. Additionally, such compounds may be effective in treating inflammatory liver disease, such as non-alcoholic steatohepatitis, reducing the likelihood of disease progression to cirrhosis or cancer.
Keywords: cannabinoid 1 receptor; diabetes; fatty liver; hepatic energy state; non-alcoholic steatohepatitis; obesity.
© 2013 The British Pharmacological Society.
Figures
Similar articles
-
CB1R regulates soluble leptin receptor levels via CHOP, contributing to hepatic leptin resistance.Elife. 2020 Nov 19;9:e60771. doi: 10.7554/eLife.60771. Elife. 2020. PMID: 33210603 Free PMC article.
-
New peripherally-restricted CB1 receptor antagonists, PMG-505-010 and -013 ameliorate obesity-associated NAFLD and fibrosis.Biomed Pharmacother. 2024 Nov;180:117501. doi: 10.1016/j.biopha.2024.117501. Epub 2024 Oct 3. Biomed Pharmacother. 2024. PMID: 39366030
-
A novel peripheral cannabinoid 1 receptor antagonist, AJ5012, improves metabolic outcomes and suppresses adipose tissue inflammation in obese mice.FASEB J. 2019 Mar;33(3):4314-4326. doi: 10.1096/fj.201801152RR. Epub 2018 Dec 19. FASEB J. 2019. PMID: 30566396
-
Role of Cannabinoid Receptor Type 1 in Insulin Resistance and Its Biological Implications.Int J Mol Sci. 2019 Apr 29;20(9):2109. doi: 10.3390/ijms20092109. Int J Mol Sci. 2019. PMID: 31035653 Free PMC article. Review.
-
Peripheral CB1R as a modulator of metabolic inflammation.FASEB J. 2021 Apr;35(4):e21232. doi: 10.1096/fj.202001960R. FASEB J. 2021. PMID: 33715173 Review.
Cited by
-
Influence of rimonabant treatment on peripheral blood mononuclear cells; flow cytometry analysis and gene expression profiling.PeerJ. 2015 Jun 30;3:e1056. doi: 10.7717/peerj.1056. eCollection 2015. PeerJ. 2015. PMID: 26157624 Free PMC article.
-
Leveraging allostery to improve G protein-coupled receptor (GPCR)-directed therapeutics: cannabinoid receptor 1 as discovery target.Expert Opin Drug Discov. 2016 Dec;11(12):1223-1237. doi: 10.1080/17460441.2016.1245289. Epub 2016 Oct 21. Expert Opin Drug Discov. 2016. PMID: 27712124 Free PMC article. Review.
-
Developmental programming of somatic growth, behavior and endocannabinoid metabolism by variation of early postnatal nutrition in a cross-fostering mouse model.PLoS One. 2017 Aug 31;12(8):e0182754. doi: 10.1371/journal.pone.0182754. eCollection 2017. PLoS One. 2017. PMID: 28859076 Free PMC article.
-
Hepatic overexpression of protein targeting to glycogen attenuates obesity and improves hyperglycemia in db/db mice.Front Endocrinol (Lausanne). 2022 Sep 9;13:969924. doi: 10.3389/fendo.2022.969924. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36157460 Free PMC article.
-
CB1 antagonism produces behaviors more consistent with satiety than reduced reward value in food-maintained responding in rats.J Psychopharmacol. 2016 May;30(5):482-91. doi: 10.1177/0269881116639287. Epub 2016 Mar 22. J Psychopharmacol. 2016. PMID: 27005309 Free PMC article.
References
-
- Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–1119. - PubMed
-
- Friedman MI. Obesity and the hepatic control of feeding behavior. Drug News Perspect. 2007;20:573–578. - PubMed
-
- Allen MS, Bradford BJ, Oba M. The hepatic oxidation theory of the control of feed intake and its application to ruminants. J Anim Sci. 2009;87:3317–3334. - PubMed
-
- Koch JE, Ji H, Osbakken MD, Friedman MI. Temporal relationships between eating behavior and liver adenine nucleotides in rats treated with 2,5-AM. Am J Physiol. 1998;274:R610–617. - PubMed
-
- Rawson NE, Friedman MI. Phosphate loading prevents the decrease in ATP and increase in food intake produced by 2,5-anhydro-D-mannitol. Am J Physiol. 1994;266:R1792–1796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

