Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers
- PMID: 23442817
- PMCID: PMC3599349
- DOI: 10.1186/1756-8722-6-19
Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers
Abstract
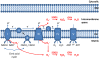
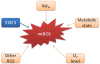
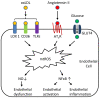
There are multiple sources of reactive oxygen species (ROS) in the cell. As a major site of ROS production, mitochondria have drawn considerable interest because it was recently discovered that mitochondrial ROS (mtROS) directly stimulate the production of proinflammatory cytokines and pathological conditions as diverse as malignancies, autoimmune diseases, and cardiovascular diseases all share common phenotype of increased mtROS production above basal levels. Several excellent reviews on this topic have been published, but ever-changing new discoveries mandated a more up-to-date and comprehensive review on this topic. Therefore, we update recent understanding of how mitochondria generate and regulate the production of mtROS and the function of mtROS both in physiological and pathological conditions. In addition, we describe newly developed methods to probe or scavenge mtROS and compare these methods in detail. Thorough understanding of this topic and the application of mtROS-targeting drugs in the research is significant towards development of better therapies to combat inflammatory diseases and inflammatory malignancies.
Figures


Similar articles
-
Mitochondrial ROS, uncoupled from ATP synthesis, determine endothelial activation for both physiological recruitment of patrolling cells and pathological recruitment of inflammatory cells.Can J Physiol Pharmacol. 2017 Mar;95(3):247-252. doi: 10.1139/cjpp-2016-0515. Epub 2016 Nov 5. Can J Physiol Pharmacol. 2017. PMID: 27925481 Free PMC article. Review.
-
Proton leak regulates mitochondrial reactive oxygen species generation in endothelial cell activation and inflammation - A novel concept.Arch Biochem Biophys. 2019 Feb 15;662:68-74. doi: 10.1016/j.abb.2018.12.002. Epub 2018 Dec 3. Arch Biochem Biophys. 2019. PMID: 30521782 Free PMC article. Review.
-
Mitochondrial Reactive Oxygen Species: Double-Edged Weapon in Host Defense and Pathological Inflammation During Infection.Front Immunol. 2020 Aug 14;11:1649. doi: 10.3389/fimmu.2020.01649. eCollection 2020. Front Immunol. 2020. PMID: 32922385 Free PMC article. Review.
-
Vicious inducible nitric oxide synthase-mitochondrial reactive oxygen species cycle accelerates inflammatory response and causes liver injury in rats.Antioxid Redox Signal. 2015 Mar 1;22(7):572-86. doi: 10.1089/ars.2014.5996. Epub 2014 Dec 22. Antioxid Redox Signal. 2015. PMID: 25365698
-
A chlorogenic acid-phospholipid complex ameliorates post-myocardial infarction inflammatory response mediated by mitochondrial reactive oxygen species in SAMP8 mice.Pharmacol Res. 2018 Apr;130:110-122. doi: 10.1016/j.phrs.2018.01.006. Epub 2018 Feb 4. Pharmacol Res. 2018. PMID: 29408518
Cited by
-
MicroRNA-21 Lowers Blood Pressure in Spontaneous Hypertensive Rats by Upregulating Mitochondrial Translation.Circulation. 2016 Sep 6;134(10):734-51. doi: 10.1161/CIRCULATIONAHA.116.023926. Epub 2016 Aug 19. Circulation. 2016. PMID: 27542393 Free PMC article.
-
Long COVID-19 and the Heart: Is Cardiac Mitochondria the Missing Link?Antioxid Redox Signal. 2023 Mar;38(7-9):599-618. doi: 10.1089/ars.2022.0126. Epub 2022 Dec 28. Antioxid Redox Signal. 2023. PMID: 36053670 Free PMC article. Review.
-
Effect of magnesium sulfate in oxidized lipid bilayers properties by using molecular dynamics.Biochem Biophys Rep. 2021 Apr 28;26:100998. doi: 10.1016/j.bbrep.2021.100998. eCollection 2021 Jul. Biochem Biophys Rep. 2021. PMID: 33997315 Free PMC article.
-
ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes.Redox Biol. 2020 Oct;37:101696. doi: 10.1016/j.redox.2020.101696. Epub 2020 Aug 27. Redox Biol. 2020. PMID: 32950427 Free PMC article. Review.
-
Dynamics of Human Mitochondrial Complex I Assembly: Implications for Neurodegenerative Diseases.Front Mol Biosci. 2016 Aug 22;3:43. doi: 10.3389/fmolb.2016.00043. eCollection 2016. Front Mol Biosci. 2016. PMID: 27597947 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

