Identification of a novel protein-protein interaction motif mediating interaction of GPCR-associated sorting proteins with G protein-coupled receptors
- PMID: 23441177
- PMCID: PMC3575409
- DOI: 10.1371/journal.pone.0056336
Identification of a novel protein-protein interaction motif mediating interaction of GPCR-associated sorting proteins with G protein-coupled receptors
Abstract
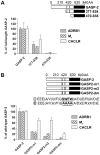
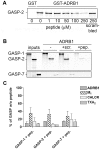
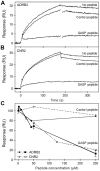
GPCR desensitization and down-regulation are considered key molecular events underlying the development of tolerance in vivo. Among the many regulatory proteins that are involved in these complex processes, GASP-1 have been shown to participate to the sorting of several receptors toward the degradation pathway. This protein belongs to the recently identified GPCR-associated sorting proteins (GASPs) family that comprises ten members for which structural and functional details are poorly documented. We present here a detailed structure-function relationship analysis of the molecular interaction between GASPs and a panel of GPCRs. In a first step, GST-pull down experiments revealed that all the tested GASPs display significant interactions with a wide range of GPCRs. Importantly, the different GASP members exhibiting the strongest interaction properties were also characterized by the presence of a small, highly conserved and repeated "GASP motif" of 15 amino acids. We further showed using GST-pull down, surface plasmon resonance and co-immunoprecipitation experiments that the central domain of GASP-1, which contains 22 GASP motifs, is essential for the interaction with GPCRs. We then used site directed mutagenesis and competition experiments with synthetic peptides to demonstrate that the GASP motif, and particularly its highly conserved core sequence SWFW, is critically involved in the interaction with GPCRs. Overall, our data show that several members of the GASP family interact with GPCRs and highlight the presence within GASPs of a novel protein-protein interaction motif that might represent a new target to investigate the involvement of GASPs in the modulation of the activity of GPCRs.
Conflict of interest statement
Figures

Similar articles
-
Identification of a novel family of G protein-coupled receptor associated sorting proteins.J Neurochem. 2004 May;89(3):766-75. doi: 10.1111/j.1471-4159.2004.02411.x. J Neurochem. 2004. PMID: 15086532
-
Characterization of anti-GASP motif antibodies that inhibit the interaction between GPRASP1 and G protein-coupled receptors.Anal Biochem. 2023 Mar 15;665:115062. doi: 10.1016/j.ab.2023.115062. Epub 2023 Jan 31. Anal Biochem. 2023. PMID: 36731712
-
The GPCR-associated sorting protein 1 regulates ligand-induced down-regulation of GPR55.Br J Pharmacol. 2012 Apr;165(8):2611-9. doi: 10.1111/j.1476-5381.2011.01562.x. Br J Pharmacol. 2012. PMID: 21718301 Free PMC article.
-
Identification and biological significance of G protein-coupled receptor associated sorting proteins (GASPs).Pharmacol Ther. 2010 Jun;126(3):244-50. doi: 10.1016/j.pharmthera.2010.03.004. Epub 2010 Apr 13. Pharmacol Ther. 2010. PMID: 20394773 Review.
-
G protein-coupled receptor-associated sorting proteins: function and relevant disorders.Yi Chuan. 2020 Aug 20;42(8):713-724. doi: 10.16288/j.yczz.20-020. Yi Chuan. 2020. PMID: 32952108 Review.
Cited by
-
Novel small molecules targeting ciliary transport of Smoothened and oncogenic Hedgehog pathway activation.Sci Rep. 2016 Mar 2;6:22540. doi: 10.1038/srep22540. Sci Rep. 2016. PMID: 26931153 Free PMC article.
-
Computational Framework for Prediction of Peptide Sequences That May Mediate Multiple Protein Interactions in Cancer-Associated Hub Proteins.PLoS One. 2016 May 24;11(5):e0155911. doi: 10.1371/journal.pone.0155911. eCollection 2016. PLoS One. 2016. PMID: 27218803 Free PMC article.
-
G protein-coupled receptors: what a difference a 'partner' makes.Int J Mol Sci. 2014 Jan 16;15(1):1112-42. doi: 10.3390/ijms15011112. Int J Mol Sci. 2014. PMID: 24441568 Free PMC article. Review.
-
Gαs regulates the post-endocytic sorting of G protein-coupled receptors.Nat Commun. 2014 Aug 4;5:4556. doi: 10.1038/ncomms5556. Nat Commun. 2014. PMID: 25089012 Free PMC article.
-
14-3-3 Proteins and Other Candidates form Protein-Protein Interactions with the Cytosolic C-terminal End of SOS1 Affecting Its Transport Activity.Int J Mol Sci. 2020 May 8;21(9):3334. doi: 10.3390/ijms21093334. Int J Mol Sci. 2020. PMID: 32397251 Free PMC article.
References
-
- Ferguson SS (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53: 1–24. - PubMed
-
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M (1999) A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 401: 286–290. - PubMed
-
- Li JG, Chen C, Liu-Chen LY (2002) Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate. J Biol Chem 277: 27545–27552. - PubMed
-
- Cong M, Perry SJ, Hu LA, Hanson PI, Claing A, et al. (2001) Binding of the beta2 adrenergic receptor to N-ethylmaleimide-sensitive factor regulates receptor recycling. J Biol Chem 276: 45145–45152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

