New insights into DNA recognition by zinc fingers revealed by structural analysis of the oncoprotein ZNF217
- PMID: 23436653
- PMCID: PMC3624442
- DOI: 10.1074/jbc.M112.441451
New insights into DNA recognition by zinc fingers revealed by structural analysis of the oncoprotein ZNF217
Abstract
Classical zinc fingers (ZFs) are one of the most abundant and best characterized DNA-binding domains. Typically, tandem arrays of three or more ZFs bind DNA target sequences with high affinity and specificity, and the mode of DNA recognition is sufficiently well understood that tailor-made ZF-based DNA-binding proteins can be engineered. We have shown previously that a two-zinc finger unit found in the transcriptional coregulator ZNF217 recognizes DNA but with an affinity and specificity that is lower than other ZF arrays. To investigate the basis for these differences, we determined the structure of a ZNF217-DNA complex. We show that although the overall position of the ZFs on the DNA closely resembles that observed for other ZFs, the side-chain interaction pattern differs substantially from the canonical model. The structure also reveals the presence of two methyl-π interactions, each featuring a tyrosine contacting a thymine methyl group. To our knowledge, interactions of this type have not previously been described in classical ZF-DNA complexes. Finally, we investigated the sequence specificity of this two-ZF unit and discuss how ZNF217 might discriminate its target DNA sites in the cell.
Figures
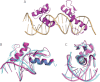
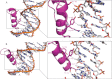
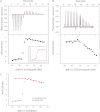

Similar articles
-
Structural metal sites in nonclassical zinc finger proteins involved in transcriptional and translational regulation.Acc Chem Res. 2014 Aug 19;47(8):2643-50. doi: 10.1021/ar500182d. Epub 2014 Aug 6. Acc Chem Res. 2014. PMID: 25098749
-
The multi-zinc finger protein ZNF217 contacts DNA through a two-finger domain.J Biol Chem. 2011 Nov 4;286(44):38190-38201. doi: 10.1074/jbc.M111.301234. Epub 2011 Sep 11. J Biol Chem. 2011. PMID: 21908891 Free PMC article.
-
Specific recognition of ZNF217 and other zinc finger proteins at a surface groove of C-terminal binding proteins.Mol Cell Biol. 2006 Nov;26(21):8159-72. doi: 10.1128/MCB.00680-06. Epub 2006 Aug 28. Mol Cell Biol. 2006. PMID: 16940172 Free PMC article.
-
Updated understanding of the protein-DNA recognition code used by C2H2 zinc finger proteins.Curr Opin Struct Biol. 2024 Aug;87:102836. doi: 10.1016/j.sbi.2024.102836. Epub 2024 May 15. Curr Opin Struct Biol. 2024. PMID: 38754172 Review.
-
Amplification of zinc finger gene 217 (ZNF217) and cancer: when good fingers go bad.Biochim Biophys Acta. 2007 Jun;1775(2):333-40. doi: 10.1016/j.bbcan.2007.05.001. Epub 2007 May 22. Biochim Biophys Acta. 2007. PMID: 17572303 Review.
Cited by
-
Structural basis for interaction between CLAMP and MSL2 proteins involved in the specific recruitment of the dosage compensation complex in Drosophila.Nucleic Acids Res. 2022 Jun 24;50(11):6521-6531. doi: 10.1093/nar/gkac455. Nucleic Acids Res. 2022. PMID: 35648444 Free PMC article.
-
Discrete-State Kinetics Model for NMR-Based Analysis of Protein Translocation on DNA at Equilibrium.J Phys Chem B. 2017 Oct 19;121(41):9548-9556. doi: 10.1021/acs.jpcb.7b07779. Epub 2017 Oct 4. J Phys Chem B. 2017. PMID: 28922916 Free PMC article.
-
DNA recognition of 5-carboxylcytosine by a Zfp57 mutant at an atomic resolution of 0.97 Å.Biochemistry. 2013 Dec 23;52(51):9310-7. doi: 10.1021/bi401360n. Epub 2013 Nov 20. Biochemistry. 2013. PMID: 24236546 Free PMC article.
-
Promiscuous DNA-binding of a mutant zinc finger protein corrupts the transcriptome and diminishes cell viability.Nucleic Acids Res. 2017 Feb 17;45(3):1130-1143. doi: 10.1093/nar/gkw1014. Nucleic Acids Res. 2017. PMID: 28180284 Free PMC article.
-
DNA methylation and de-methylation using hybrid site-targeting proteins.Genome Biol. 2018 Nov 6;19(1):187. doi: 10.1186/s13059-018-1566-2. Genome Biol. 2018. PMID: 30400938 Free PMC article. Review.
References
-
- Klug A., Schwabe J. W. (1995) Protein motifs 5. Zinc fingers. FASEB J. 9, 597–604 - PubMed
-
- Mackay J. P., Crossley M. (1998) Zinc fingers are sticking together. Trends Biochem. Sci. 23, 1–4 - PubMed
-
- Sánchez-García I., Rabbitts T. H. (1994) The LIM domain: a new structural motif found in zinc-finger-like proteins. Trends Genet. 10, 315–320 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

