Directional spread of alphaherpesviruses in the nervous system
- PMID: 23435239
- PMCID: PMC3640521
- DOI: 10.3390/v5020678
Directional spread of alphaherpesviruses in the nervous system
Abstract
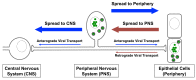
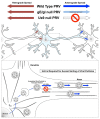
Alphaherpesviruses are pathogens that invade the nervous systems of their mammalian hosts. Directional spread of infection in the nervous system is a key component of the viral lifecycle and is critical for the onset of alphaherpesvirus-related diseases. Many alphaherpesvirus infections originate at peripheral sites, such as epithelial tissues, and then enter neurons of the peripheral nervous system (PNS), where lifelong latency is established. Following reactivation from latency and assembly of new viral particles, the infection typically spreads back out towards the periphery. These spread events result in the characteristic lesions (cold sores) commonly associated with herpes simplex virus (HSV) and herpes zoster (shingles) associated with varicella zoster virus (VZV). Occasionally, the infection spreads transsynaptically from the PNS into higher order neurons of the central nervous system (CNS). Spread of infection into the CNS, while rarer in natural hosts, often results in severe consequences, including death. In this review, we discuss the viral and cellular mechanisms that govern directional spread of infection in the nervous system. We focus on the molecular events that mediate long distance directional transport of viral particles in neurons during entry and egress.
Figures
Similar articles
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
-
Recent Advances in the Study of Alphaherpesvirus Latency and Reactivation: Novel Guidance for the Design of Herpesvirus Live Vector Vaccines.Pathogens. 2024 Sep 10;13(9):779. doi: 10.3390/pathogens13090779. Pathogens. 2024. PMID: 39338969 Free PMC article. Review.
-
Herpesvirus transport to the nervous system and back again.Annu Rev Microbiol. 2012;66:153-76. doi: 10.1146/annurev-micro-092611-150051. Epub 2012 Jun 15. Annu Rev Microbiol. 2012. PMID: 22726218 Free PMC article. Review.
-
Dual-Color Herpesvirus Capsids Discriminate Inoculum from Progeny and Reveal Axonal Transport Dynamics.J Virol. 2016 Oct 14;90(21):9997-10006. doi: 10.1128/JVI.01122-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27581973 Free PMC article.
-
Microtubule-Dependent Trafficking of Alphaherpesviruses in the Nervous System: The Ins and Outs.Viruses. 2019 Dec 17;11(12):1165. doi: 10.3390/v11121165. Viruses. 2019. PMID: 31861082 Free PMC article. Review.
Cited by
-
In vivo dynamics of AAV-mediated gene delivery to sensory neurons of the trigeminal ganglia.Sci Rep. 2017 Apr 19;7(1):927. doi: 10.1038/s41598-017-01004-y. Sci Rep. 2017. PMID: 28424485 Free PMC article.
-
Inner tegument proteins of Herpes Simplex Virus are sufficient for intracellular capsid motility in neurons but not for axonal targeting.PLoS Pathog. 2017 Dec 28;13(12):e1006813. doi: 10.1371/journal.ppat.1006813. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29284065 Free PMC article.
-
A pUL25 dimer interfaces the pseudorabies virus capsid and tegument.J Gen Virol. 2017 Nov;98(11):2837-2849. doi: 10.1099/jgv.0.000903. Epub 2017 Oct 16. J Gen Virol. 2017. PMID: 29035172 Free PMC article.
-
High-brightness anterograde transneuronal HSV1 H129 tracer modified using a Trojan horse-like strategy.Mol Brain. 2020 Jan 13;13(1):5. doi: 10.1186/s13041-020-0544-2. Mol Brain. 2020. PMID: 31931837 Free PMC article.
-
Herpes simplex virus-1 evasion of CD8+ T cell accumulation contributes to viral encephalitis.J Clin Invest. 2017 Oct 2;127(10):3784-3795. doi: 10.1172/JCI92931. Epub 2017 Sep 11. J Clin Invest. 2017. PMID: 28891812 Free PMC article.
References
-
- Pellett P.E., Roizman B. The Family: Herpesviridae a Brief Introduction. In: Knipe D.M., Howley P.M., Griffin D.E., Lamb R.A., Martin M.A., Roizman B., Straus S.E., editors. Fields Virology. 5th. Vol. 2. Lippincott, Williams, and Wilkins; Philadelphia, PA, USA: 2007. pp. 2479–2500.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

