Contribution of α7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure
- PMID: 23431157
- PMCID: PMC3593882
- DOI: 10.1073/pnas.1216939110
Contribution of α7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure
Abstract
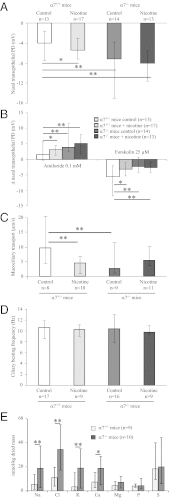
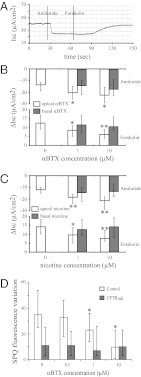
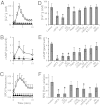
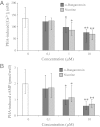
Loss or dysfunction of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) leads to impairment of airway mucus transport and to chronic lung diseases resulting in progressive respiratory failure. Nicotinic acetylcholine receptors (nAChRs) bind nicotine and nicotine-derived nitrosamines and thus mediate many of the tobacco-related deleterious effects in the lung. Here we identify α7 nAChR as a key regulator of CFTR in the airways. The airway epithelium in α7 knockout mice is characterized by a higher transepithelial potential difference, an increase of amiloride-sensitive apical Na(+) absorption, a defective cAMP-dependent Cl(-) conductance, higher concentrations of Na(+), Cl(-), K(+), and Ca(2+) in secretions, and a decreased mucus transport, all relevant to a deficient CFTR activity. Moreover, prolonged nicotine exposure mimics the absence of α7 nAChR in mice or its inactivation in vitro in human airway epithelial cell cultures. The functional coupling of α7 nAChR to CFTR occurs through Ca(2+) entry and activation of adenylyl cyclases, protein kinase A, and PKC. α7 nAChR, CFTR, and adenylyl cyclase-1 are physically and functionally associated in a macromolecular complex within lipid rafts at the apical membrane of surface and glandular airway epithelium. This study establishes the potential role of α7 nAChR in the regulation of CFTR function and in the pathogenesis of smoking-related chronic lung diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Evidence for functional atypical nicotinic receptors that activate K+-dependent Cl- secretion in mouse tracheal epithelium.Am J Respir Cell Mol Biol. 2012 Jan;46(1):106-14. doi: 10.1165/rcmb.2011-0171OC. Am J Respir Cell Mol Biol. 2012. PMID: 21852683
-
{alpha}7 nicotinic acetylcholine receptor regulates airway epithelium differentiation by controlling basal cell proliferation.Am J Pathol. 2009 Nov;175(5):1868-82. doi: 10.2353/ajpath.2009.090212. Epub 2009 Oct 1. Am J Pathol. 2009. PMID: 19808646 Free PMC article.
-
Liquid movement across the surface epithelium of large airways.Respir Physiol Neurobiol. 2007 Dec 15;159(3):256-70. doi: 10.1016/j.resp.2007.06.005. Epub 2007 Jun 17. Respir Physiol Neurobiol. 2007. PMID: 17692578 Free PMC article. Review.
-
Absence of alpha7-containing neuronal nicotinic acetylcholine receptors does not prevent nicotine-induced seizures.Brain Res Mol Brain Res. 2002 Jan 31;98(1-2):29-40. doi: 10.1016/s0169-328x(01)00309-6. Brain Res Mol Brain Res. 2002. PMID: 11834293
-
Pharmacotherapy of the ion transport defect in cystic fibrosis: role of purinergic receptor agonists and other potential therapeutics.Am J Respir Med. 2003;2(4):299-309. doi: 10.1007/BF03256658. Am J Respir Med. 2003. PMID: 14719996 Review.
Cited by
-
The Cholinergic Pathways in Inflammation: A Potential Pharmacotherapeutic Target for COPD.Front Pharmacol. 2018 Dec 3;9:1426. doi: 10.3389/fphar.2018.01426. eCollection 2018. Front Pharmacol. 2018. PMID: 30559673 Free PMC article. Review.
-
Cystic fibrosis transmembrane conductance regulator-emerging regulator of cancer.Cell Mol Life Sci. 2018 May;75(10):1737-1756. doi: 10.1007/s00018-018-2755-6. Epub 2018 Feb 6. Cell Mol Life Sci. 2018. PMID: 29411041 Free PMC article. Review.
-
Acquired Cystic Fibrosis Transmembrane Conductance Regulator Dysfunction in Chronic Bronchitis and Other Diseases of Mucus Clearance.Clin Chest Med. 2016 Mar;37(1):147-58. doi: 10.1016/j.ccm.2015.11.003. Epub 2015 Dec 24. Clin Chest Med. 2016. PMID: 26857776 Free PMC article. Review.
-
Nicotinic alpha 7 receptor expression and modulation of the lung epithelial response to lipopolysaccharide.PLoS One. 2017 Apr 6;12(4):e0175367. doi: 10.1371/journal.pone.0175367. eCollection 2017. PLoS One. 2017. PMID: 28384302 Free PMC article.
-
Evaluating Commercially Available Antibodies for Rat α7 Nicotinic Acetylcholine Receptors.J Histochem Cytochem. 2017 Sep;65(9):499-512. doi: 10.1369/0022155417725304. Epub 2017 Aug 1. J Histochem Cytochem. 2017. PMID: 28763248 Free PMC article.
References
-
- Barnes PJ. New therapies for chronic obstructive pulmonary disease. Med Princ Pract. 2010;19(5):330–338. - PubMed
-
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23(1):146–158. - PubMed
-
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

