Effect of reduced agalsidase Beta dosage in fabry patients: the Australian experience
- PMID: 23430871
- PMCID: PMC3509855
- DOI: 10.1007/8904_2011_44
Effect of reduced agalsidase Beta dosage in fabry patients: the Australian experience
Abstract
Background: In Australia, enzyme replacement therapy (ERT) for Fabry Disease (FD), both Agalsidase alfa (Replagal, Shire HGT) and beta (Fabrazyme, Genzyme), is funded and monitored through a specific government program. Agalsidase beta supply has been rationed by Genzyme since 2009 due to manufacturing issues. Consequently, the Australian Fabry Disease Advisory Committee has treated patients on Agalsidase beta at 50% of their usual dose from mid-2009, with a further reduction to 30% for some patients from late 2009.
Aim: To determine the clinical effect of Agalsidase beta dose reduction in the Australian FD patient cohort.
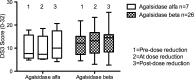
Methods: A questionnaire assessing FD symptoms was administered to 40 patients on long-term ERT. Clinical data from The Fabry Registry for patients receiving Agalsidase alfa or beta, for at least 2 years prior to the time of enforced Agalsidase beta dose reduction, were reviewed. Disease burden and quality of life (QOL) were graded using the Disease Severity Scoring System, Mainz Severity Score Index, Brief Pain Inventory and Short Form 36 Health Survey at 2 years before dose reduction, at the time of dose reduction and at the most recent clinical review following dose reduction.
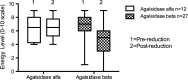
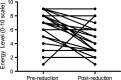
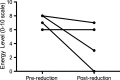
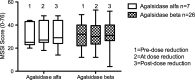
Results: Disease severity and QOL scores did not change between the ERT groups. Males on Agalsidase beta reported lower energy levels after dose reduction, while no change was reported by females on either product or by males on a stable dose of Agalsidase alfa.
Conclusion: This study suggests that energy levels in male patients worsen after dose reduction of Agalsidase beta.
Figures
Similar articles
-
Clinical observations on enzyme replacement therapy in patients with Fabry disease and the switch from agalsidase beta to agalsidase alfa.J Chin Med Assoc. 2014 Apr;77(4):190-7. doi: 10.1016/j.jcma.2013.11.006. Epub 2013 Dec 30. J Chin Med Assoc. 2014. PMID: 24388678
-
Agalsidase alfa: a review of its use in the management of Fabry disease.BioDrugs. 2012 Oct 1;26(5):335-54. doi: 10.2165/11209690-000000000-00000. BioDrugs. 2012. PMID: 22946754 Review.
-
Effects of switching from agalsidase Beta to agalsidase alfa in 10 patients with anderson-fabry disease.JIMD Rep. 2013;9:41-48. doi: 10.1007/8904_2012_177. Epub 2012 Oct 21. JIMD Rep. 2013. PMID: 23430546 Free PMC article.
-
Enzyme replacement therapy for Anderson-Fabry disease.Cochrane Database Syst Rev. 2016 Jul 25;7(7):CD006663. doi: 10.1002/14651858.CD006663.pub4. Cochrane Database Syst Rev. 2016. PMID: 27454104 Free PMC article. Review.
-
Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg.PLoS One. 2007 Jul 11;2(7):e598. doi: 10.1371/journal.pone.0000598. PLoS One. 2007. PMID: 17622343 Free PMC article.
Cited by
-
Patient and observer reported outcome measures to evaluate health-related quality of life in inherited metabolic diseases: a scoping review.Orphanet J Rare Dis. 2018 Nov 28;13(1):215. doi: 10.1186/s13023-018-0953-9. Orphanet J Rare Dis. 2018. PMID: 30486833 Free PMC article.
-
Fabry nephropathy: a review - how can we optimize the management of Fabry nephropathy?BMC Nephrol. 2014 May 6;15:72. doi: 10.1186/1471-2369-15-72. BMC Nephrol. 2014. PMID: 24886109 Free PMC article. Review.
-
The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: A systematic literature review by a European panel of experts.Mol Genet Metab Rep. 2019 Feb 6;19:100454. doi: 10.1016/j.ymgmr.2019.100454. eCollection 2019 Jun. Mol Genet Metab Rep. 2019. PMID: 30775256 Free PMC article. Review.
-
The prevalence and epidemiology of genetic renal disease amongst adults with chronic kidney disease in Australia.Orphanet J Rare Dis. 2014 Jun 30;9:98. doi: 10.1186/1750-1172-9-98. Orphanet J Rare Dis. 2014. PMID: 24980890 Free PMC article.
-
Dose-Finding Studies Among Orphan Drugs Approved in the EU: A Retrospective Analysis.J Clin Pharmacol. 2019 Feb;59(2):229-244. doi: 10.1002/jcph.1304. Epub 2018 Sep 7. J Clin Pharmacol. 2019. PMID: 30192386 Free PMC article.
References
-
- Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, van Roomen C, Mirzaian M, Wijburg FA, Linthorst GE, Vedder AC, Rombach SM, Cox-Brinkman J, Somerharju P, Boot RG, Hollak CE, Brady RO, Poorthuis BJ. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008;105(8):2812–2817. doi: 10.1073/pnas.0712309105. - DOI - PMC - PubMed
-
- Aerts JM, Kallemeijn WW, Wegdam W, Joao Ferraz M, van Breemen MJ, Dekker N, Kramer G, Poorthuis BJ, Groener JE, Cox-Brinkman J, Rombach SM, Hollak CE, Linthorst GE, Witte MD, Gold H, van der Marel GA, Overkleeft HS, Boot RG. Biomarkers in the diagnosis of lysosomal storage disorders: proteins, lipids, and inhibodies. J Inherit Metab Dis. 2011;34:605–619. doi: 10.1007/s10545-011-9308-6. - DOI - PMC - PubMed
-
- Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, Finkel R, Packman S, Bichet DG, Warnock DG, Desnick RJ. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med. 2007;146(2):77–86. - PubMed
LinkOut - more resources
Full Text Sources

