The degree of Helicobacter pylori-triggered inflammation is manipulated by preinfection host microbiota
- PMID: 23429529
- PMCID: PMC3647981
- DOI: 10.1128/IAI.00044-13
The degree of Helicobacter pylori-triggered inflammation is manipulated by preinfection host microbiota
Abstract
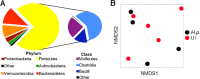
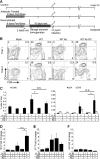
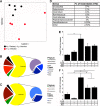
Helicobacter pylori infects over 3 billion people worldwide and is the primary risk factor for gastric cancer. Most individuals infected with H. pylori develop only asymptomatic gastritis; however, some develop ulcers or gastric adenocarcinoma. We demonstrate that one previously unappreciated parameter influencing H. pylori disease outcome is variation in the preinfection host microbiota. Utilizing a mouse model, we altered the microbiota by antibiotic treatment and found that these alterations resulted in significantly lowered H. pylori-triggered inflammation. Specifically, antibiotic pretreatment reduced CD4(+) T-helper cells and Ifnγ transcript levels in gastric tissue after H. pylori infection. The bacterial communities in mice with a reduced response to H. pylori displayed many differences from those in untreated mice, including significantly more cluster IV and XIVa Clostridium spp., bacteria known to influence inflammation via regulatory T cell populations. Our findings suggest that microbiota composition, perhaps Clostridium spp., contributes to the variable disease outcome of H. pylori infection by altering the recruitment of CD4(+) T cells to the gastric compartment. Our results suggest that gastric microbiota could be used as a diagnostic tool to determine which patients are at risk for developing severe disease.
Figures

Similar articles
-
A pro-inflammatory role for Th22 cells in Helicobacter pylori-associated gastritis.Gut. 2015 Sep;64(9):1368-78. doi: 10.1136/gutjnl-2014-307020. Epub 2014 Aug 18. Gut. 2015. PMID: 25134787 Free PMC article.
-
CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo.Gastroenterology. 2006 Aug;131(2):525-37. doi: 10.1053/j.gastro.2006.05.001. Gastroenterology. 2006. PMID: 16890606
-
Changes in gastric microbiota induced by Helicobacter pylori infection and preventive effects of Lactobacillus plantarum ZDY 2013 against such infection.J Dairy Sci. 2016 Feb;99(2):970-981. doi: 10.3168/jds.2015-10510. Epub 2015 Dec 17. J Dairy Sci. 2016. PMID: 26709179
-
Impairment of ghrelin synthesis in Helicobacter pylori-colonized stomach: new clues for the pathogenesis of H. pylori-related gastric inflammation.World J Gastroenterol. 2014 Jan 21;20(3):639-46. doi: 10.3748/wjg.v20.i3.639. World J Gastroenterol. 2014. PMID: 24574737 Free PMC article. Review.
-
Immune responses to Helicobacter pylori infection.World J Gastroenterol. 2014 May 21;20(19):5583-93. doi: 10.3748/wjg.v20.i19.5583. World J Gastroenterol. 2014. PMID: 24914318 Free PMC article. Review.
Cited by
-
Helicobacter pylori, gastric microbiota and gastric cancer relationship: Unrolling the tangle.World J Gastrointest Oncol. 2022 May 15;14(5):959-972. doi: 10.4251/wjgo.v14.i5.959. World J Gastrointest Oncol. 2022. PMID: 35646287 Free PMC article. Review.
-
Impact of Helicobacter pylori eradication on the gastric microbiome.Gut Pathog. 2021 Oct 13;13(1):60. doi: 10.1186/s13099-021-00460-2. Gut Pathog. 2021. PMID: 34645495 Free PMC article.
-
Helicobacter pylori is associated with increased risk of serrated colonic polyps: Analysis of serrated polyp risk factors.Indian J Gastroenterol. 2018 May;37(3):235-242. doi: 10.1007/s12664-018-0855-8. Epub 2018 Jun 7. Indian J Gastroenterol. 2018. PMID: 29876742
-
Exploring the Link between Helicobacter pylori, Gastric Microbiota and Gastric Cancer.Antibiotics (Basel). 2024 May 24;13(6):484. doi: 10.3390/antibiotics13060484. Antibiotics (Basel). 2024. PMID: 38927151 Free PMC article. Review.
-
Effects of Proton Pump Inhibitors on the Gastric Mucosa-Associated Microbiota in Dyspeptic Patients.Appl Environ Microbiol. 2016 Oct 27;82(22):6633-6644. doi: 10.1128/AEM.01437-16. Print 2016 Nov 15. Appl Environ Microbiol. 2016. PMID: 27590821 Free PMC article.
References
-
- Atherton JC. 2006. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu. Rev. Pathol. 1:63–96 - PubMed
-
- Oertli M, Sundquist M, Hitzler I, Engler DB, Arnold IC, Reuter S, Maxeiner J, Hansson M, Taube C, Quiding-Järbrink M, Müller A. 2012. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori--specific immune tolerance, and asthma protection. J. Clin. Invest. 122:1082–1096 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

