Antigen choice determines vaccine-induced generation of immunogenic versus tolerogenic dendritic cells that are marked by differential expression of pancreatic enzymes
- PMID: 23420890
- PMCID: PMC3608825
- DOI: 10.4049/jimmunol.1203321
Antigen choice determines vaccine-induced generation of immunogenic versus tolerogenic dendritic cells that are marked by differential expression of pancreatic enzymes
Abstract
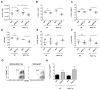
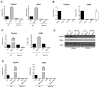
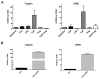
Dendritic cells (DC) elicit immunity to pathogens and tumors while simultaneously preserving tolerance to self. Efficacious cancer vaccines have been a challenge because they are based on tumor Ags, some of which are self-Ags and thus subject to self-tolerance. One such Ag is the tumor-associated mucin MUC1. Preclinical testing of MUC1 vaccines revealed existence of peripheral tolerance to MUC1 that compromises their efficacy. To identify mechanisms that act early postvaccination and might predict vaccine outcome, we immunized human MUC1 transgenic mice (MUC1.Tg) i.v. with a MUC1 peptide vaccine against which they generate weak immunity and wild-type (WT) mice that respond strongly to the same peptide. We analyzed differences in splenic DC phenotype and function between the two mouse strains at 24 and 72 h postvaccination and also performed unbiased total gene expression analysis of the spleen. Compared to WT, MUC1.Tg spleens had significantly fewer DC, and they exhibited significantly lower expression of costimulatory molecules, decreased motility, and preferential priming of Ag-specific Foxp3(+) regulatory T cells. This tolerogenic DC phenotype and function was marked by a new putative biomarker revealed by the microarray: a cohort of pancreatic enzymes (trypsin, carboxypeptidase, elastase, and others) not previously reported in DC. These enzymes were strongly upregulated in the splenic DC from vaccinated WT mice and suppressed in the splenic DC of vaccinated MUC1.Tg mice. Suppression of the enzymes was dependent on regulatory T cells and on signaling through the IL-10R and correlated with global downregulation of DC immunostimulatory phenotype and function.
Figures
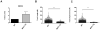
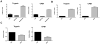
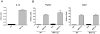
Similar articles
-
Novel mechanisms underlying the immediate and transient global tolerization of splenic dendritic cells after vaccination with a self-antigen.J Immunol. 2014 Jan 15;192(2):658-65. doi: 10.4049/jimmunol.1301904. Epub 2013 Dec 11. J Immunol. 2014. PMID: 24337381 Free PMC article.
-
Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection.J Immunol. 2001 Jun 1;166(11):6555-63. doi: 10.4049/jimmunol.166.11.6555. J Immunol. 2001. PMID: 11359807
-
Vaccination with allogeneic dendritic cells fused to carcinoma cells induces antitumor immunity in MUC1 transgenic mice.Clin Immunol. 2001 Nov;101(2):192-200. doi: 10.1006/clim.2001.5112. Clin Immunol. 2001. PMID: 11683578
-
Lack of effective MUC1 tumor antigen-specific immunity in MUC1-transgenic mice results from a Th/T regulatory cell imbalance that can be corrected by adoptive transfer of wild-type Th cells.J Immunol. 2007 Mar 1;178(5):2787-93. doi: 10.4049/jimmunol.178.5.2787. J Immunol. 2007. PMID: 17312122
-
Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA.J Immunol. 2000 Nov 15;165(10):5713-9. doi: 10.4049/jimmunol.165.10.5713. J Immunol. 2000. PMID: 11067929
Cited by
-
Global Inhibition of DC Priming Capacity in the Spleen of Self-Antigen Vaccinated Mice Requires IL-10.Front Immunol. 2014 Feb 17;5:59. doi: 10.3389/fimmu.2014.00059. eCollection 2014. Front Immunol. 2014. PMID: 24596571 Free PMC article.
-
Targeting of anti-citrullinated protein/peptide antibodies in rheumatoid arthritis using peptides mimicking endogenously citrullinated fibrinogen antigens.Arthritis Res Ther. 2015 Jun 10;17(1):155. doi: 10.1186/s13075-015-0666-6. Arthritis Res Ther. 2015. PMID: 26059223 Free PMC article.
-
Engineered tissues and strategies to overcome challenges in drug development.Adv Drug Deliv Rev. 2020;158:116-139. doi: 10.1016/j.addr.2020.09.012. Epub 2020 Sep 26. Adv Drug Deliv Rev. 2020. PMID: 32987094 Free PMC article.
-
Novel mechanisms underlying the immediate and transient global tolerization of splenic dendritic cells after vaccination with a self-antigen.J Immunol. 2014 Jan 15;192(2):658-65. doi: 10.4049/jimmunol.1301904. Epub 2013 Dec 11. J Immunol. 2014. PMID: 24337381 Free PMC article.
-
Fifty Shades of Tolerance: Beyond a Binary Tolerant/Non-Tolerant Paradigm.Curr Transplant Rep. 2017 Dec;4(4):262-269. doi: 10.1007/s40472-017-0166-5. Epub 2017 Oct 6. Curr Transplant Rep. 2017. PMID: 31098340 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

