Energy metabolism and energy-sensing pathways in mammalian embryonic and adult stem cell fate
- PMID: 23420198
- PMCID: PMC3575699
- DOI: 10.1242/jcs.114827
Energy metabolism and energy-sensing pathways in mammalian embryonic and adult stem cell fate
Abstract
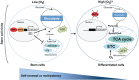
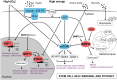
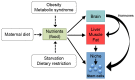
Metabolism is influenced by age, food intake, and conditions such as diabetes and obesity. How do physiological or pathological metabolic changes influence stem cells, which are crucial for tissue homeostasis? This Commentary reviews recent evidence that stem cells have different metabolic demands than differentiated cells, and that the molecular mechanisms that control stem cell self-renewal and differentiation are functionally connected to the metabolic state of the cell and the surrounding stem cell niche. Furthermore, we present how energy-sensing signaling molecules and metabolism regulators are implicated in the regulation of stem cell self-renewal and differentiation. Finally, we discuss the emerging literature on the metabolism of induced pluripotent stem cells and how manipulating metabolic pathways might aid cellular reprogramming. Determining how energy metabolism regulates stem cell fate should shed light on the decline in tissue regeneration that occurs during aging and facilitate the development of therapies for degenerative or metabolic diseases.
Figures
Similar articles
-
Interplay between Metabolites and the Epigenome in Regulating Embryonic and Adult Stem Cell Potency and Maintenance.Stem Cell Reports. 2019 Oct 8;13(4):573-589. doi: 10.1016/j.stemcr.2019.09.003. Stem Cell Reports. 2019. PMID: 31597110 Free PMC article. Review.
-
Burn to cycle: energetics of cell-cycle control and stem cell maintenance.Front Biosci (Landmark Ed). 2014 Jun 1;19(6):1003-14. doi: 10.2741/4263. Front Biosci (Landmark Ed). 2014. PMID: 24896332 Review.
-
Energy metabolism in the acquisition and maintenance of stemness.Semin Cell Dev Biol. 2016 Apr;52:68-75. doi: 10.1016/j.semcdb.2016.02.010. Epub 2016 Feb 8. Semin Cell Dev Biol. 2016. PMID: 26868758 Free PMC article. Review.
-
Connecting Mitochondria, Metabolism, and Stem Cell Fate.Stem Cells Dev. 2015 Sep 1;24(17):1957-71. doi: 10.1089/scd.2015.0117. Epub 2015 Jul 2. Stem Cells Dev. 2015. PMID: 26134242 Free PMC article. Review.
-
TGF-β Family Signaling in Embryonic and Somatic Stem-Cell Renewal and Differentiation.Cold Spring Harb Perspect Biol. 2017 Jul 5;9(7):a022186. doi: 10.1101/cshperspect.a022186. Cold Spring Harb Perspect Biol. 2017. PMID: 28108485 Free PMC article. Review.
Cited by
-
Ex vivo AKT-inhibition facilitates generation of polyfunctional stem cell memory-like CD8+ T cells for adoptive immunotherapy.Oncoimmunology. 2018 Aug 6;7(10):e1488565. doi: 10.1080/2162402X.2018.1488565. eCollection 2018. Oncoimmunology. 2018. PMID: 30288356 Free PMC article.
-
PFKFB3-mediated glycometabolism reprogramming modulates endothelial differentiation and angiogenic capacity of placenta-derived mesenchymal stem cells.Stem Cell Res Ther. 2022 Aug 2;13(1):391. doi: 10.1186/s13287-022-03089-3. Stem Cell Res Ther. 2022. PMID: 35918720 Free PMC article.
-
The Epithelial-Mesenchymal Transition at the Crossroads between Metabolism and Tumor Progression.Int J Mol Sci. 2022 Jan 12;23(2):800. doi: 10.3390/ijms23020800. Int J Mol Sci. 2022. PMID: 35054987 Free PMC article. Review.
-
Adult Stem Cells and Diseases of Aging.J Clin Med. 2014 Jan 21;3(1):88-134. doi: 10.3390/jcm3010088. J Clin Med. 2014. PMID: 24757526 Free PMC article.
-
Metabolic control of primed human pluripotent stem cell fate and function by the miR-200c-SIRT2 axis.Nat Cell Biol. 2017 May;19(5):445-456. doi: 10.1038/ncb3517. Epub 2017 Apr 24. Nat Cell Biol. 2017. PMID: 28436968 Free PMC article.
References
-
- Banko M. R., Allen J. J., Schaffer B. E., Wilker E. W., Tsou P., White J. L., Villén J., Wang B., Kim S. R., Sakamoto K.et al. (2011). Chemical genetic screen for AMPKα2 substrates uncovers a network of proteins involved in mitosis. Mol. Cell 44, 878–892 10.1016/j.molcel.2011.11.005 - DOI - PMC - PubMed
-
- Bonnert T. P., Bilsland J. G., Guest P. C., Heavens R., McLaren D., Dale C., Thakur M., McAllister G., Munoz–Sanjuan I. (2006). Molecular characterization of adult mouse subventricular zone progenitor cells during the onset of differentiation. Eur. J. Neurosci. 24, 661–675 10.1111/j.1460-9568.2006.04912.x - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

