Vesicle-mediated export from the ER: COPII coat function and regulation
- PMID: 23419775
- PMCID: PMC3676692
- DOI: 10.1016/j.bbamcr.2013.02.003
Vesicle-mediated export from the ER: COPII coat function and regulation
Abstract
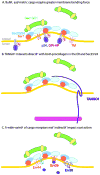
Vesicle trafficking from the endoplasmic reticulum (ER) is a vital cellular process in all eukaryotes responsible for moving secretory cargoes from the ER to the Golgi apparatus. To accomplish this feat, the cell employs a set of conserved cytoplasmic coat proteins - the coat protein II (COPII) complex - that recruit cargo into nascent buds and deform the ER membrane to drive vesicle formation. While our understanding of COPII coat mechanics has developed substantially since its discovery, we have only recently begun to appreciate the factors that regulate this complex and, in turn, ER-to-Golgi trafficking. Here, we describe these factors and their influences on COPII vesicle formation. Properties intrinsic to the GTP cycle of the coat, as well as coat structure, have critical implications for COPII vesicle trafficking. Extrinsic factors in the cytosol can modulate COPII activity through direct interaction with the coat or with scaffolding components, or by changing composition of the ER membrane. Further, lumenal and membrane-bound cargoes and cargo receptors can influence COPII-mediated trafficking in equally profound ways. Together, these factors work in concert to ensure proper cargo movement in this first step of the secretory pathway. This article is part of a Special Issue entitled: Functional and structural diversity of endoplasmic reticulum.
Keywords: CLSD; COPII; Cargo export; Cargo receptor; ER; ER exit sites; ERES; ERK; Endoplasmic reticulum; GAP; GEF; GPI-AP; GST; GTPase activating protein; MAPK; PH; PtdIns4P; TANGO1; TFG-1; TRK-fused gene 1; VSVG; Vesicle; bst; bypass-of-sec-thirteen; cTAGE5; coat protein II complex; cranio–lentinculo–sutural dysplasia; cutaneous T-cell lymphoma-associated antigen 5; endoplasmic reticulum; extracellular signal-regulated kinase; glutathione S-transferase; glycosylphophatidylinositol-anchored protein; guanine nucleotide exchange factor; mitogen activated protein kinase; phosphatidylinositol 4-phosphate; pleckstrin homology; transport and Golgi organization 1; vesicular stomatitis virus G protein.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
COPII-mediated trafficking at the ER/ERGIC interface.Traffic. 2019 Jul;20(7):491-503. doi: 10.1111/tra.12654. Epub 2019 May 30. Traffic. 2019. PMID: 31059169 Free PMC article. Review.
-
COPII collar defines the boundary between ER and ER exit site and does not coat cargo containers.J Cell Biol. 2021 Jun 7;220(6):e201907224. doi: 10.1083/jcb.201907224. J Cell Biol. 2021. PMID: 33852719 Free PMC article.
-
TFG facilitates outer coat disassembly on COPII transport carriers to promote tethering and fusion with ER-Golgi intermediate compartments.Proc Natl Acad Sci U S A. 2017 Sep 12;114(37):E7707-E7716. doi: 10.1073/pnas.1709120114. Epub 2017 Aug 29. Proc Natl Acad Sci U S A. 2017. PMID: 28851831 Free PMC article.
-
COPII coat assembly and selective export from the endoplasmic reticulum.J Biochem. 2004 Dec;136(6):755-60. doi: 10.1093/jb/mvh184. J Biochem. 2004. PMID: 15671485 Review.
-
TFG regulates inner COPII coat recruitment to facilitate anterograde secretory protein transport.Mol Biol Cell. 2024 Aug 1;35(8):ar113. doi: 10.1091/mbc.E24-06-0282. Epub 2024 Jul 10. Mol Biol Cell. 2024. PMID: 38985515 Free PMC article.
Cited by
-
Transcriptional regulation of secretory capacity by bZip transcription factors.Front Biol (Beijing). 2015 Feb 1;10(1):28-51. doi: 10.1007/s11515-014-1338-7. Front Biol (Beijing). 2015. PMID: 25821458 Free PMC article.
-
Distinct stages in the recognition, sorting, and packaging of proTGFα into COPII-coated transport vesicles.Mol Biol Cell. 2016 Jun 15;27(12):1938-47. doi: 10.1091/mbc.E16-02-0090. Epub 2016 Apr 27. Mol Biol Cell. 2016. PMID: 27122606 Free PMC article.
-
Silencing of Small Valosin-containing Protein-interacting Protein (SVIP) Reduces Very Low Density Lipoprotein (VLDL) Secretion from Rat Hepatocytes by Disrupting Its Endoplasmic Reticulum (ER)-to-Golgi Trafficking.J Biol Chem. 2016 Jun 10;291(24):12514-12526. doi: 10.1074/jbc.M115.705269. Epub 2016 Apr 15. J Biol Chem. 2016. PMID: 27129256 Free PMC article.
-
A virtuous cycle operated by ERp44 and ERGIC-53 guarantees proteostasis in the early secretory compartment.iScience. 2021 Mar 1;24(3):102244. doi: 10.1016/j.isci.2021.102244. eCollection 2021 Mar 19. iScience. 2021. PMID: 33763635 Free PMC article.
-
Comparison of the Agronomic, Cytological, Grain Protein Characteristics, as Well as Transcriptomic Profile of Two Wheat Lines Derived From Wild Emmer.Front Genet. 2022 Jan 28;12:804481. doi: 10.3389/fgene.2021.804481. eCollection 2021. Front Genet. 2022. PMID: 35154252 Free PMC article.
References
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed
-
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. - PubMed
-
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nature cell biology. 2001;3:531–537. - PubMed
-
- Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. - PubMed
-
- Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2012;14:20–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

