Norspermidine and novel Pd(II) and Pt(II) polynuclear complexes of norspermidine as potential antineoplastic agents against breast cancer
- PMID: 23418450
- PMCID: PMC3572109
- DOI: 10.1371/journal.pone.0055651
Norspermidine and novel Pd(II) and Pt(II) polynuclear complexes of norspermidine as potential antineoplastic agents against breast cancer
Abstract
Background: New strategies are needed for breast cancer treatment and one initial step is to test new chemotherapeutic drugs in breast cancer cell lines, to choose candidates for further studies towards clinical use.
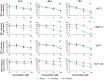
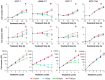
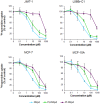
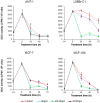
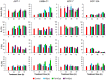
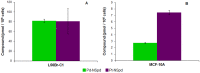
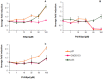
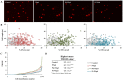
Methodology and findings: The cytotoxic effects of a biogenic polyamine analogue - norspermidine - and its trinuclear Pd(II) and Pt(II) complexes - Pd(3)NSpd(2) and Pt(3)NSpd(2), respectively - were investigated in one immortalized normal-like and three breast cancer cell lines. The normal-like MCF-10A cells were least sensitive to the compounds, while growth inhibition and cell death was observed in the cancer cell lines. Norspermidine and its Pd(II) complex were generally shown to have stronger antiproliferative effects than the corresponding Pt(II) complex. Moreover, both norspermidine and the Pd(II) complex reduced the cellular activity of the growth-related enzyme, ornithine decarboxylase (ODC) to a lower level than the Pt(II) complex in most of the cell lines examined. Treatment with norspermidine or the Pd(II) complex reduced the number of colonies formed in a soft agar assay performed with the breast cancer cell lines, indicating that these compounds reduced the malignancy of the breast cancer cells. The effect of norspermidine or the Pd(II) complex on colony formation was much stronger than that observed for the Pt(II) complex. The results from a new mammalian genotoxicity screen together with those of a single cell gel electrophoresis assay indicated that none of the drugs were genotoxic at a 25 µM concentration.
Main conclusions: Overall, norspermidine and its Pd(II) complex were shown to have strong antiproliferative effects. In comparison, the effects obtained with the Pd(II) complex were much stronger than that of the Pt(II) complex. The results obtained in the present study demonstrate that the trinuclear Pd(II) complex of norspermidine (Pd(3)NSpd(2)) may be regarded as a potential new metal-based drug against breast cancer, coupling a significant efficiency to a low toxicity.
Conflict of interest statement
Figures
Similar articles
-
Antitumor activity of norspermidine, a structural homologue of the natural polyamine spermidine.Anticancer Res. 1988 Jul-Aug;8(4):563-8. Anticancer Res. 1988. PMID: 3140710
-
Novel Pt(II) and Pd(II) complexes with polyamine analogues: synthesis and vibrational analysis.J Inorg Biochem. 2012 Mar;108:1-7. doi: 10.1016/j.jinorgbio.2011.11.021. Epub 2011 Dec 3. J Inorg Biochem. 2012. PMID: 22265832 Free PMC article.
-
Biochemical and growth-modulatory effects of the new S-adenosylmethionine decarboxylase inhibitor CGP 48664 in malignant and immortalized normal human breast epithelial cells in culture.Int J Cancer. 1995 Aug 9;62(4):485-91. doi: 10.1002/ijc.2910620421. Int J Cancer. 1995. PMID: 7635576
-
Palladium(II) and platinum(II) organometallic complexes with 4,7-dihydro-5-methyl-7-oxo[1,2,4]triazolo[1,5-a]pyrimidine. Antitumor activity of the platinum compounds.Inorg Chem. 2008 Jun 2;47(11):4490-505. doi: 10.1021/ic701873b. Epub 2008 Apr 30. Inorg Chem. 2008. PMID: 18447329
-
Characterization of Pt-, Pd-spermine complexes for their effect on polyamine pathway and cisplatin resistance in A2780 ovarian carcinoma cells.Oncol Rep. 2010 Jul;24(1):15-24. doi: 10.3892/or_00000823. Oncol Rep. 2010. PMID: 20514439 Free PMC article.
Cited by
-
Response of Osteosarcoma Cell Metabolism to Platinum and Palladium Chelates as Potential New Drugs.Molecules. 2021 Aug 8;26(16):4805. doi: 10.3390/molecules26164805. Molecules. 2021. PMID: 34443394 Free PMC article.
-
Increased breast cancer cell toxicity by palladination of the polyamine analogue N (1),N (11)-bis(ethyl)norspermine.Amino Acids. 2014 Feb;46(2):339-52. doi: 10.1007/s00726-013-1621-y. Epub 2013 Dec 21. Amino Acids. 2014. PMID: 24363201 Free PMC article.
-
On the correction of calculated vibrational frequencies for the effects of the counterions - α,ω-diamine dihydrochlorides.J Mol Model. 2015 Oct;21(10):266. doi: 10.1007/s00894-015-2818-7. Epub 2015 Sep 19. J Mol Model. 2015. PMID: 26386957
-
An Evaluation of Norspermidine on Anti-fungal Effect on Mature Candida albicans Biofilms and Angiogenesis Potential of Dental Pulp Stem Cells.Front Bioeng Biotechnol. 2020 Aug 12;8:948. doi: 10.3389/fbioe.2020.00948. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32903416 Free PMC article.
-
A novel coordination complex of platinum (PT) induces cell death in colorectal cancer by altering redox balance and modulating MAPK pathway.BMC Cancer. 2020 Jul 23;20(1):685. doi: 10.1186/s12885-020-07165-w. BMC Cancer. 2020. PMID: 32703189 Free PMC article.
References
-
- Simstein R, Burow M, Parker A, Weldon C, Beckman B (2003) Apoptosis, chemoresistance, and breast cancer: insights from the MCF-7 cell model system. Exp Biol Med (Maywood) 228: 995–1003. - PubMed
-
- Davidson NE, Hahm HA, McCloskey DE, Woster PM, Casero RA Jr (1999) Clinical aspects of cell death in breast cancer: the polyamine pathway as a new target for treatment. Endocr Relat Cancer 6: 69–73. - PubMed
-
- Decatris MP, Sundar S, O’Byrne KJ (2005) Platinum-based chemotherapy in metastatic breast cancer: the Leicester (U.K.) experience. Clin Oncol (R Coll Radiol) 17: 249–257. - PubMed
-
- Pegg AE (1988) Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res 48: 759–774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

