Lethal Crimean-Congo hemorrhagic fever virus infection in interferon α/β receptor knockout mice is associated with high viral loads, proinflammatory responses, and coagulopathy
- PMID: 23417661
- PMCID: PMC3654741
- DOI: 10.1093/infdis/jit061
Lethal Crimean-Congo hemorrhagic fever virus infection in interferon α/β receptor knockout mice is associated with high viral loads, proinflammatory responses, and coagulopathy
Abstract
Crimean-Congo hemorrhagic fever (CCHF) is a widely distributed viral hemorrhagic fever characterized by rapid onset of flu-like symptoms often followed by hemorrhagic manifestations. CCHF virus (CCHFV), a bunyavirus in the Nairovirus genus, is capable of infecting a wide range of mammalian hosts in nature but so far only causes disease in humans. Recently, immunocompromised mice have been reported as CCHF disease models, but detailed characterization is lacking. Here, we closely followed infection and disease progression in CCHFV-infected interferon α/β receptor knockout (IFNAR(-/-)) mice and age-matched wild-type (WT) mice. WT mice quickly clear CCHFV without developing any disease signs. In contrast, CCHFV infected IFNAR(-/-) mice develop an acute fulminant disease with high viral loads leading to organ pathology (liver and lymphoid tissues), marked proinflammatory host responses, severe thrombocytopenia, coagulopathy, and death. Disease progression closely mimics hallmarks of human CCHF disease, making IFNAR(-/-) mice an excellent choice to assess medical countermeasures.
Keywords: CCHFV; coagulopathy; interferon α/β receptor knockout mice; pathology; proinflammatory response; thrombocytopenia.
Figures
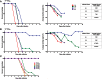


Similar articles
-
Crimean-Congo Hemorrhagic Fever Mouse Model Recapitulating Human Convalescence.J Virol. 2019 Aug 28;93(18):e00554-19. doi: 10.1128/JVI.00554-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31292241 Free PMC article.
-
Nucleoside-Modified mRNA Vaccines Protect IFNAR-/- Mice against Crimean-Congo Hemorrhagic Fever Virus Infection.J Virol. 2022 Feb 9;96(3):e0156821. doi: 10.1128/JVI.01568-21. Epub 2021 Nov 24. J Virol. 2022. PMID: 34817199 Free PMC article.
-
Hazara virus infection is lethal for adult type I interferon receptor-knockout mice and may act as a surrogate for infection with the human-pathogenic Crimean-Congo hemorrhagic fever virus.J Gen Virol. 2012 Mar;93(Pt 3):560-564. doi: 10.1099/vir.0.038455-0. Epub 2011 Nov 16. J Gen Virol. 2012. PMID: 22090213
-
Crimean-Congo hemorrhagic fever.Antiviral Res. 2004 Dec;64(3):145-60. doi: 10.1016/j.antiviral.2004.08.001. Antiviral Res. 2004. PMID: 15550268 Review.
-
A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus.Antiviral Res. 2016 Nov;135:31-47. doi: 10.1016/j.antiviral.2016.09.013. Epub 2016 Oct 3. Antiviral Res. 2016. PMID: 27713073 Free PMC article. Review.
Cited by
-
A cynomolgus macaque model for Crimean-Congo haemorrhagic fever.Nat Microbiol. 2018 May;3(5):556-562. doi: 10.1038/s41564-018-0141-7. Epub 2018 Apr 9. Nat Microbiol. 2018. PMID: 29632370 Free PMC article.
-
Second International Conference on Crimean-Congo Hemorrhagic Fever.Antiviral Res. 2018 Feb;150:137-147. doi: 10.1016/j.antiviral.2017.11.019. Epub 2017 Dec 2. Antiviral Res. 2018. PMID: 29199036 Free PMC article.
-
ISG15 overexpression compensates the defect of Crimean-Congo hemorrhagic fever virus polymerase bearing a protease-inactive ovarian tumor domain.PLoS Negl Trop Dis. 2020 Sep 15;14(9):e0008610. doi: 10.1371/journal.pntd.0008610. eCollection 2020 Sep. PLoS Negl Trop Dis. 2020. PMID: 32931521 Free PMC article.
-
Favipiravir (T-705) but not ribavirin is effective against two distinct strains of Crimean-Congo hemorrhagic fever virus in mice.Antiviral Res. 2018 Sep;157:18-26. doi: 10.1016/j.antiviral.2018.06.013. Epub 2018 Jun 21. Antiviral Res. 2018. PMID: 29936152 Free PMC article.
-
Systems-level temporal immune-metabolic profile in Crimean-Congo hemorrhagic fever virus infection.Proc Natl Acad Sci U S A. 2023 Sep 12;120(37):e2304722120. doi: 10.1073/pnas.2304722120. Epub 2023 Sep 5. Proc Natl Acad Sci U S A. 2023. PMID: 37669378 Free PMC article.
References
-
- Whitehouse CA. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004;64:145–60. - PubMed
-
- Vorou R, Pierroutsakos IN, Maltezou HC. Crimean-Congo hemorrhagic fever. Curr Opin Infect Dis. 2007;20:495–500. - PubMed
-
- Shepherd AJ, Leman PA, Swanepoel R. Viremia and antibody response of small African and laboratory animals to Crimean-Congo hemorrhagic fever virus infection. Am J Trop Med Hyg. 1989;40:541–7. - PubMed
-
- Shepherd AJ, Swanepoel R, Cornel AJ, Mathee O. Experimental studies on the replication and transmission of Crimean-Congo hemorrhagic fever virus in some African tick species. Am J Trop Med Hyg. 1989;40:326–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

