Pain after discontinuation of morphine treatment is associated with synaptic increase of GluA4-containing AMPAR in the dorsal horn of the spinal cord
- PMID: 23403695
- PMCID: PMC3682142
- DOI: 10.1038/npp.2013.46
Pain after discontinuation of morphine treatment is associated with synaptic increase of GluA4-containing AMPAR in the dorsal horn of the spinal cord
Abstract
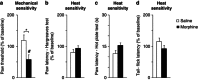
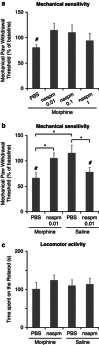
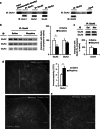
Withdrawal from prescribed opioids results in increased pain sensitivity, which prolongs the treatment. This pain sensitivity is attributed to neuroplastic changes that converge at the spinal cord dorsal horn. We have recently reported that repeated morphine administration triggers an insertion of GluA2-lacking (Ca(2+)-permeable) α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPAR) in the hippocampus. This finding together with the reported involvement of AMPAR in the mechanisms underlying inflammatory pain led us to hypothesize a role for spinal AMPAR in opioid-induced pain behavior. Mice treated with escalating doses of morphine showed hypersensitivity to mechanical stimulation. Intrathecal administration of a Ca(2+)-permeable AMPAR selective blocker disrupted morphine-induced mechanical sensitivity. Analysis of the expression and phosphorylation levels of AMPAR subunits (GluA1/2/3/4) in homogenates and in postsynaptic density fractions from spinal cord dorsal horns showed an increase in GluA4 expression and phosphorylation in the postsynaptic density after morphine. Co-immunoprecipitation analyses suggested an increase in GluA4 homomers (Ca(2+)-permeable AMPAR) and immunohistochemical staining localized the increase in GluA4 levels in laminae III-V. The excitatory postsynaptic currents (EPSCs) recorded in laminae III-V showed enhanced sensitivity to Ca(2+)-permeable AMPAR blockers in morphine-treated mice. Furthermore, current-voltage relationships of AMPAR-mediated EPSCs showed that rectification index (an indicator of Ca(2+)-permeable AMPAR contribution) is increased in morphine-treated but not in saline-treated mice. These effects could be reversed by infusion of GluA4 antibody through patch pipette. This is the first direct evidence for a role of GluA4-containing AMPAR in morphine-induced pain and highlights spinal GluA4-containing AMPAR as targets to prevent the morphine-induced pain sensitivity.
Figures


Similar articles
-
Opioid receptors inhibit the spinal AMPA receptor Ca2+ permeability that mediates latent pain sensitization.Exp Neurol. 2019 Apr;314:58-66. doi: 10.1016/j.expneurol.2019.01.003. Epub 2019 Jan 17. Exp Neurol. 2019. PMID: 30660616 Free PMC article.
-
Inflammation-induced GluA1 trafficking and membrane insertion of Ca2+ permeable AMPA receptors in dorsal horn neurons is dependent on spinal tumor necrosis factor, PI3 kinase and protein kinase A.Exp Neurol. 2017 Jul;293:144-158. doi: 10.1016/j.expneurol.2017.04.004. Epub 2017 Apr 12. Exp Neurol. 2017. PMID: 28412220 Free PMC article.
-
Nerve injury increases GluA2-lacking AMPA receptor prevalence in spinal cords: functional significance and signaling mechanisms.J Pharmacol Exp Ther. 2013 Dec;347(3):765-72. doi: 10.1124/jpet.113.208363. Epub 2013 Sep 12. J Pharmacol Exp Ther. 2013. PMID: 24030012 Free PMC article.
-
Extrasynaptic AMPA receptors in the dorsal horn: evidence and functional significance.Brain Res Bull. 2013 Apr;93:47-56. doi: 10.1016/j.brainresbull.2012.11.004. Epub 2012 Nov 26. Brain Res Bull. 2013. PMID: 23194665 Review.
-
Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain.Anesthesiology. 2010 May;112(5):1259-65. doi: 10.1097/ALN.0b013e3181d3e1ed. Anesthesiology. 2010. PMID: 20395828 Free PMC article. Review.
Cited by
-
Neuron Type-Dependent Synaptic Activity in the Spinal Dorsal Horn of Opioid-Induced Hyperalgesia Mouse Model.Front Synaptic Neurosci. 2021 Nov 18;13:748929. doi: 10.3389/fnsyn.2021.748929. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34867259 Free PMC article.
-
Glioma synapses recruit mechanisms of adaptive plasticity.Nature. 2023 Nov;623(7986):366-374. doi: 10.1038/s41586-023-06678-1. Epub 2023 Nov 1. Nature. 2023. PMID: 37914930 Free PMC article.
-
Identification of an epidermal keratinocyte AMPA glutamate receptor involved in dermatopathies associated with sensory abnormalities.Pain Rep. 2016 Sep;1(3):e573. doi: 10.1097/PR9.0000000000000573. Pain Rep. 2016. PMID: 28210712 Free PMC article.
-
In vivo activation of the SK channel in the spinal cord reduces the NMDA receptor antagonist dose needed to produce antinociception in an inflammatory pain model.Pain. 2015 May;156(5):849-858. doi: 10.1097/j.pain.0000000000000124. Pain. 2015. PMID: 25734988 Free PMC article.
-
What Is Being Trained? How Divergent Forms of Plasticity Compete To Shape Locomotor Recovery after Spinal Cord Injury.J Neurotrauma. 2017 May 15;34(10):1831-1840. doi: 10.1089/neu.2016.4562. Epub 2017 Jan 13. J Neurotrauma. 2017. PMID: 27875927 Free PMC article. Review.
References
-
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. - PubMed
-
- Billa SK, Liu J, Bjorklund NL, Sinha N, Fu Y, Shinnick-Gallagher P, et al. Increased insertion of glutamate receptor 2-lacking alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors at hippocampal synapses upon repeated morphine administration. Mol Pharmacol. 2010;77:874–883. - PMC - PubMed
-
- Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochem Soc Trans. 2005;33:1354–1356. - PubMed
-
- Buldakova SL, Kim KK, Tikhonov DB, Magazanik LG. Selective blockade of Ca2+ permeable AMPA receptors in CA1 area of rat hippocampus. Neuroscience. 2007;144:88–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

