Polarization and symmetry of electronic transitions in long fluorescence lifetime triangulenium dyes
- PMID: 23391292
- PMCID: PMC3600155
- DOI: 10.1021/jp312376k
Polarization and symmetry of electronic transitions in long fluorescence lifetime triangulenium dyes
Abstract
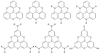
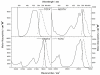
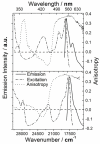
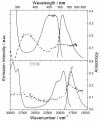
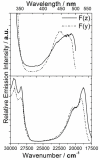
To fully exploit the capabilities of fluorescence probes in modern experiments, where advanced instrumentation is used to probe complex environments, other photophysical properties than emission color and emission intensity are monitored. Each dye property can be addressed individually as well as collectively to provide in-depth information unavailable from the standard intensity measurements. Dyes with long emission lifetimes and strongly polarized transitions enable the monitoring of lifetime changes as well as emission polarization (anisotropy). Thus experiments can be designed to follow slow dynamics. The UV and visible electronic transitions of a series of red-emitting dyes based on the triangulenium motif are investigated. We resolve overlapping features in the spectra and assign the orientation of the transition moments to the molecular axes. The result is the complete Jablonski diagram for the UV and visible spectral region. The symmetries of the studied dyes are shown to have a large influence on the optical response, and they are clearly separated into two groups of symmetry by their photophysical properties. The C(2v) symmetric dyes, azadioxatriangulenium (ADOTA(+)) and diazaoxatriangulenium (DAOTA(+)), have high emission anisotropies, fluorescence lifetimes around 20 ns, and fluorescence quantum yields of ∼50%. The trioxatriangulenium (TOTA(+)) and triazatriangulenium (TATA(+)) dyes-nominally of D(3h) symmetry-have fluorescence lifetimes around 10 ns lifetimes and fluorescence quantum yields of 10-15%. However, the D(3h) symmetry is shown to be lowered to a point group, where the axes transform uniquely such that the degeneracy of the E' states is lifted.
Figures

Similar articles
-
Azadioxatriangulenium and Diazaoxatriangulenium: Quantum Yields and Fundamental Photophysical Properties.ACS Omega. 2017 Jan 24;2(1):193-203. doi: 10.1021/acsomega.6b00211. eCollection 2017 Jan 31. ACS Omega. 2017. PMID: 31457221 Free PMC article.
-
Long-lived bright red emitting azaoxa-triangulenium fluorophores.PLoS One. 2013 May 7;8(5):e63043. doi: 10.1371/journal.pone.0063043. Print 2013. PLoS One. 2013. PMID: 23667570 Free PMC article.
-
Synthesis and optical properties of trioxatriangulenium dyes with one and two peripheral amino substituents.J Org Chem. 2010 Sep 17;75(18):6182-90. doi: 10.1021/jo1009917. J Org Chem. 2010. PMID: 20738142
-
Azadioxatriangulenium: Synthesis and Photophysical Properties of Reactive Dyes for Bioconjugation.European J Org Chem. 2015 Oct;2015(28):6351-6358. doi: 10.1002/ejoc.201500888. European J Org Chem. 2015. PMID: 27047257 Free PMC article.
-
Mini review of ultrafast fluorescence polarization spectroscopy [invited].Appl Opt. 2013 Feb 10;52(5):917-29. doi: 10.1364/AO.52.000917. Appl Opt. 2013. PMID: 23400053 Review.
Cited by
-
Azadioxatriangulenium and Diazaoxatriangulenium: Quantum Yields and Fundamental Photophysical Properties.ACS Omega. 2017 Jan 24;2(1):193-203. doi: 10.1021/acsomega.6b00211. eCollection 2017 Jan 31. ACS Omega. 2017. PMID: 31457221 Free PMC article.
-
Determining Excited-State Absorption Properties of a Quinoid Flavin by Polarization-Resolved Transient Spectroscopy.J Phys Chem A. 2024 May 16;128(19):3830-3839. doi: 10.1021/acs.jpca.4c01260. Epub 2024 May 6. J Phys Chem A. 2024. PMID: 38709806 Free PMC article.
-
C-Functionalized Cationic Diazaoxatriangulenes: Late-Stage Synthesis and Tuning of Physicochemical Properties.Chemistry. 2018 Jul 17;24(40):10186-10195. doi: 10.1002/chem.201801486. Epub 2018 Jun 25. Chemistry. 2018. PMID: 29698563 Free PMC article.
-
Covalent and non-covalent coupling of a Au102 nanocluster with a fluorophore: energy transfer, quenching and intracellular pH sensing.Nanoscale Adv. 2021 Sep 24;3(23):6649-6658. doi: 10.1039/d1na00368b. eCollection 2021 Nov 24. Nanoscale Adv. 2021. PMID: 36132657 Free PMC article.
-
Long-lived bright red emitting azaoxa-triangulenium fluorophores.PLoS One. 2013 May 7;8(5):e63043. doi: 10.1371/journal.pone.0063043. Print 2013. PLoS One. 2013. PMID: 23667570 Free PMC article.
References
-
- Schäfer FP, Drexhage KH. Dye lasers. Springer-Verlag; Berlin, New York: 1973. p. 285. p x.
- Johnson I. Fluorescent probes for living cells. Histochem.J. 1998;30(3):123–140. - PubMed
- Stephens DJ, Allan VJ. Light microscopy techniques for live cell Imaging. Science. 2003;300(5616):82–86. - PubMed
- Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. Review - The fluorescent toolbox for assessing protein location and function. Science. 2006;312(5771):217–224. - PubMed
- Lakowicz JR. Principles of Fluorescence Spectroscopy
- Loudet A, Burgess K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007;107(11):4891–4932. - PubMed
- Lavis LD, Raines RT. Bright ideas for chemical biology. ACS Chem. Bio. 2008;3(3):142–155. - PMC - PubMed
- McDonagh C, Burke CS, MacCraith BD. Optical chemical sensors. Chem. Rev. 2008;108(2):400–422. - PubMed
- Sreejith S, Carol P, Chithra P, Ajayaghosh A. Squaraine dyes: a mine of molecular materials. J. Mater. Chem. 2008;18(3):264–274.
- Ulrich G, Ziessel R, Harriman A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008;47(7):1184–1201. - PubMed
-
- Lewis G, Calvin M. The Color of Organic Substances. Chem. Rev. 1939;25(2):273–328.
- Martin JC, Smith RG. Factors Influencing Basicities of Triarylcarbinols Synthesis of Sesquixanthydrol. J. Am. Chem. Soc. 1964;86(11):2252–2256.
- Seybold G, Wagenblast G. New Perylene and Violanthrone Dyestuffs for Fluorescent Collectors. Dyes and Pigments. 1989;11:303–317.
- Turro NJ. Modern Molecular Photochemistry. 1 ed. University Science Books; 1991. p. 627.
-
- Whitaker JE, Haugland RP, Prendergast FG. Spectral and Photophysical Studies of Benzo[c]xanthene Dyes: Dual Emission pH Sensors. Anal. Biochem. 1991;194:330–344. - PubMed
- Umezawa K, Matsui A, Nakamura Y, Citterio D, Suzuki K. Bright, Color-Tunable Fluorescent Dyes in the Vis/NIR Region: Establishment of New “Tailor-Made” Multicolor Fluorophores Based on Borondipyrromethene. Chem. Eur. J. 2009;15(5):1096–1106. - PubMed
- Haugland RP, Spence MTZ, Johnson ID. Handbook of fluorescent probes and research chemicals. 6th ed. Molecular Probes; Eugene, OR, USA: 1996. p. 680. 4849 Pitchford Ave., Eugene 97402. p xii.
-
- Seybold G, Wagenblast G. New Perylene and Violanthrone Dyestuff for Fluorescent Collector. Dyes and Pigment. 1989;11(4):303–317.
-
- Doody MA, Baker GA, Pandey S, Bright FV. Affinity and mobility of polyclonal anti-dansyl antibodies sequestered within sol-gel-derived biogels. Chem. Mater. 2000;12(4):1142–1147.
- Michl J, Eggers JH. Polarization Spectra in Stretched Polymer Sheets 4: Benzo[k]Fluoranthene and its Aza Analogs. Tetrahedron. 1974;30:813–817.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

