Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline
- PMID: 23390169
- PMCID: PMC3589296
- DOI: 10.1212/WNL.0b013e318283bb0a
Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline
Abstract
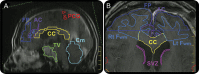
Objective: In a retrospective review to assess neuroanatomical targets of radiation-induced cognitive decline, dose volume histogram (DVH) analyses of specific brain regions of interest (ROI) are correlated to neurocognitive performance in 57 primary brain tumor survivors.
Methods: Neurocognitive assessment at baseline included Trail Making Tests A/B, a modified Rey-Osterreith Complex Figure, California or Hopkins Verbal Learning Test, Digit Span, and Controlled Oral Word Association. DVH analysis was performed for multiple neuroanatomical targets considered to be involved in cognition. The %v10 (percent of ROI receiving 10 Gy), %v40, and %v60 were calculated for each ROI. Factor analysis was used to estimate global cognition based on a summary of performance on individual cognitive tests. Stepwise regression was used to determine which dose volume predicted performance on global factors and individual neurocognitive tests for each ROI.
Results: Regions that predicted global cognitive outcomes at doses <60 Gy included the corpus callosum, left frontal white matter, right temporal lobe, bilateral hippocampi, subventricular zone, and cerebellum. Regions of adult neurogenesis primarily predicted cognition at %v40 except for the right hippocampus which predicted at %v10. Regions that did not predict global cognitive outcomes at any dose include total brain volume, frontal pole, anterior cingulate, right frontal white matter, and the right precentral gyrus.
Conclusions: Modeling of radiation-induced cognitive decline using neuroanatomical target theory appears to be feasible. A prospective trial is necessary to validate these data.
Figures
Similar articles
-
Taste and smell disturbances after brain irradiation: a dose-volume histogram analysis of a prospective observational study.Pract Radiat Oncol. 2014 Mar-Apr;4(2):130-135. doi: 10.1016/j.prro.2013.06.003. Epub 2013 Jul 25. Pract Radiat Oncol. 2014. PMID: 24890354 Free PMC article.
-
Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up.Lancet Neurol. 2009 Sep;8(9):810-8. doi: 10.1016/S1474-4422(09)70204-2. Epub 2009 Aug 7. Lancet Neurol. 2009. PMID: 19665931
-
A prospective evaluation of whole brain volume loss and neurocognitive decline following hippocampal-sparing prophylactic cranial irradiation for limited-stage small-cell lung cancer.J Neurooncol. 2019 Sep;144(2):351-358. doi: 10.1007/s11060-019-03235-7. Epub 2019 Jul 13. J Neurooncol. 2019. PMID: 31302830
-
Radiation-induced cognitive impairment--from bench to bedside.Neuro Oncol. 2012 Sep;14 Suppl 4(Suppl 4):iv37-44. doi: 10.1093/neuonc/nos196. Neuro Oncol. 2012. PMID: 23095829 Free PMC article. Review.
-
Radiation injury and neurogenesis.Curr Opin Neurol. 2003 Apr;16(2):129-34. doi: 10.1097/01.wco.0000063772.81810.b7. Curr Opin Neurol. 2003. PMID: 12644738 Review.
Cited by
-
Mild cognitive impairment in long-term brain tumor survivors following brain irradiation.J Neurooncol. 2019 Jan;141(1):235-244. doi: 10.1007/s11060-018-03032-8. Epub 2018 Nov 7. J Neurooncol. 2019. PMID: 30406339 Free PMC article.
-
Does Stereotactic Radiosurgery Have a Role in the Management of Patients Presenting With 4 or More Brain Metastases?Neurosurgery. 2019 Mar 1;84(3):558-566. doi: 10.1093/neuros/nyy216. Neurosurgery. 2019. PMID: 29860451 Free PMC article. Review.
-
Brain Microstructural Changes Associated With Neurocognitive Outcome in Intracranial Germ Cell Tumor Survivors.Front Oncol. 2021 May 26;11:573798. doi: 10.3389/fonc.2021.573798. eCollection 2021. Front Oncol. 2021. PMID: 34164332 Free PMC article.
-
Differential expression of Homer1a in the hippocampus and cortex likely plays a role in radiation-induced brain injury.Radiat Res. 2014 Jan;181(1):21-32. doi: 10.1667/RR13475.1. Epub 2013 Dec 30. Radiat Res. 2014. PMID: 24377717 Free PMC article.
-
Review of Conventional and High Dose Rate Brain Radiation (FLASH): Neurobehavioural, Neurocognitive and Assessment Issues in Rodent Models.Clin Oncol (R Coll Radiol). 2021 Nov;33(11):e482-e491. doi: 10.1016/j.clon.2021.09.002. Epub 2021 Sep 20. Clin Oncol (R Coll Radiol). 2021. PMID: 34548203 Free PMC article. Review.
References
-
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–122 - PubMed
-
- Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 1995;31:1093–1112 - PubMed
-
- Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999;45:323–329 - PubMed
-
- Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810–821 - PubMed
-
- Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys 1980;6:1215–1228 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
