Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry
- PMID: 23388071
- PMCID: PMC3599262
- DOI: 10.1186/1743-8977-10-2
Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry
Abstract
Background: The uptake of nanoparticles (NPs) by cells remains to be better characterized in order to understand the mechanisms of potential NP toxicity as well as for a reliable risk assessment. Real NP uptake is still difficult to evaluate because of the adsorption of NPs on the cellular surface.
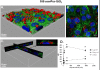
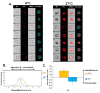
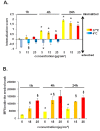
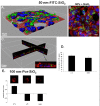
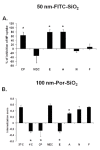
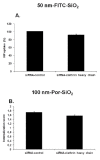
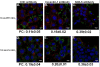
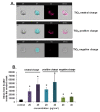
Results: Here we used two approaches to distinguish adsorbed fluorescently labeled NPs from the internalized ones. The extracellular fluorescence was either quenched by Trypan Blue or the uptake was analyzed using imaging flow cytometry. We used this novel technique to define the inside of the cell to accurately study the uptake of fluorescently labeled (SiO2) and even non fluorescent but light diffracting NPs (TiO2). Time course, dose-dependence as well as the influence of surface charges on the uptake were shown in the pulmonary epithelial cell line NCI-H292. By setting up an integrative approach combining these flow cytometric analyses with confocal microscopy we deciphered the endocytic pathway involved in SiO2 NP uptake. Functional studies using energy depletion, pharmacological inhibitors, siRNA-clathrin heavy chain induced gene silencing and colocalization of NPs with proteins specific for different endocytic vesicles allowed us to determine macropinocytosis as the internalization pathway for SiO2 NPs in NCI-H292 cells.
Conclusion: The integrative approach we propose here using the innovative imaging flow cytometry combined with confocal microscopy could be used to identify the physico-chemical characteristics of NPs involved in their uptake in view to redesign safe NPs.
Figures



Similar articles
-
Cellular uptake and intracellular localization of poly (acrylic acid) nanoparticles in a rainbow trout (Oncorhynchus mykiss) gill epithelial cell line, RTgill-W1.Aquat Toxicol. 2017 Nov;192:58-68. doi: 10.1016/j.aquatox.2017.09.008. Epub 2017 Sep 7. Aquat Toxicol. 2017. PMID: 28917946
-
Internalization of SiO₂ nanoparticles by alveolar macrophages and lung epithelial cells and its modulation by the lung surfactant substitute Curosurf.Environ Sci Pollut Res Int. 2013 May;20(5):2761-70. doi: 10.1007/s11356-012-1436-5. Epub 2013 Jan 5. Environ Sci Pollut Res Int. 2013. PMID: 23288678
-
Integrated multiplatform method for in vitro quantitative assessment of cellular uptake for fluorescent polymer nanoparticles.Nanotechnology. 2014 Jan 31;25(4):045102. doi: 10.1088/0957-4484/25/4/045102. Nanotechnology. 2014. PMID: 24398665
-
Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type.Environ Toxicol Chem. 2014 Mar;33(3):481-92. doi: 10.1002/etc.2470. Epub 2014 Jan 24. Environ Toxicol Chem. 2014. PMID: 24273100 Review.
-
Nanoparticles: cellular uptake and cytotoxicity.Adv Exp Med Biol. 2014;811:73-91. doi: 10.1007/978-94-017-8739-0_5. Adv Exp Med Biol. 2014. PMID: 24683028 Review.
Cited by
-
Ultra-small Pyropheophorbide-a Nanodots for Near-infrared Fluorescence/Photoacoustic Imaging-guided Photodynamic Therapy.Theranostics. 2020 Jan 1;10(1):62-73. doi: 10.7150/thno.35735. eCollection 2020. Theranostics. 2020. PMID: 31903106 Free PMC article.
-
Critical determinants of uptake and translocation of nanoparticles by the human pulmonary alveolar epithelium.ACS Nano. 2014 Nov 25;8(11):11778-89. doi: 10.1021/nn505399e. Epub 2014 Nov 4. ACS Nano. 2014. PMID: 25360809 Free PMC article.
-
Semi-automated quantification of living cells with internalized nanostructures.J Nanobiotechnology. 2016 Jan 15;14:4. doi: 10.1186/s12951-015-0153-x. J Nanobiotechnology. 2016. PMID: 26768888 Free PMC article.
-
Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine.Chem Soc Rev. 2021 May 7;50(9):5397-5434. doi: 10.1039/d0cs01127d. Epub 2021 Mar 5. Chem Soc Rev. 2021. PMID: 33666625 Free PMC article. Review.
-
Cationic Dendrimers for siRNA Delivery: An Overview of Methods for In Vitro/In Vivo Characterization.Methods Mol Biol. 2021;2282:209-244. doi: 10.1007/978-1-0716-1298-9_14. Methods Mol Biol. 2021. PMID: 33928579 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

