Microfluidic synthesis of PEG- and folate-conjugated liposomes for one-step formation of targeted stealth nanocarriers
- PMID: 23386106
- PMCID: PMC3650128
- DOI: 10.1007/s11095-013-0998-3
Microfluidic synthesis of PEG- and folate-conjugated liposomes for one-step formation of targeted stealth nanocarriers
Abstract
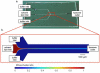
Purpose: A microfluidic hydrodynamic flow focusing technique enabling the formation of small and nearly monodisperse liposomes is investigated for continuous-flow synthesis of poly(ethylene glycol) (PEG)-modified and PEG-folate-functionalized liposomes for targeted drug delivery.
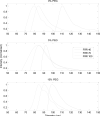
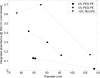
Methods: Controlled laminar flow in thermoplastic microfluidic devices facilitated liposome self-assembly from initial lipid compositions including lipid/cholesterol mixtures containing PEG-lipid and folate-PEG-lipid conjugates. Relationships among flow conditions, lipid composition, and liposome size were evaluated; their impact on PEG and folate incorporation were determined through a combination of UV-vis absorbance measurements and characterization of liposome zeta potential.
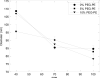
Results: PEG and folate were successfully incorporated into microfluidic-synthesized liposomes over the full range of liposome sizes studied. Efficiency of PEG-lipid incorporation was inversely correlated with liposome diameter. Folate-lipid was effectively integrated into liposomes at various flow conditions.
Conclusions: Liposomes incorporating relatively large PEG-modified and folate-PEG-modified lipids were successfully synthesized using the microfluidic flow focusing platform, providing a simple, low cost, rapid method for preparing functionalized liposomes. Relationships between preparation conditions and PEG or folate-PEG functionalization have been elucidated, providing insight into the process and defining paths for optimization of the microfluidic method toward the formation of functionalized liposomes for pharmaceutical applications.
Figures



Similar articles
-
Microfluidic preparation of liposomes to determine particle size influence on cellular uptake mechanisms.Pharm Res. 2014 Feb;31(2):401-13. doi: 10.1007/s11095-013-1171-8. Epub 2013 Oct 3. Pharm Res. 2014. PMID: 24092051
-
Folate-PEG coated cationic modified chitosan--cholesterol liposomes for tumor-targeted drug delivery.Biomaterials. 2010 May;31(14):4129-38. doi: 10.1016/j.biomaterials.2010.01.089. Epub 2010 Feb 16. Biomaterials. 2010. PMID: 20163853
-
Microfluidic synthesis of multifunctional liposomes for tumour targeting.Colloids Surf B Biointerfaces. 2016 Dec 1;148:402-410. doi: 10.1016/j.colsurfb.2016.09.016. Epub 2016 Sep 12. Colloids Surf B Biointerfaces. 2016. PMID: 27639490
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
[Effect of polyethylene glycol-lipid derivatives on the stability of grafted liposomes].Yao Xue Xue Bao. 2011 Oct;46(10):1178-86. Yao Xue Xue Bao. 2011. PMID: 22242447 Review. Chinese.
Cited by
-
Microfluidic Generation of Near-Infrared Photothermal Vitexin/ICG Liposome with Amplified Photodynamic Therapy.AAPS PharmSciTech. 2023 Mar 22;24(4):82. doi: 10.1208/s12249-023-02539-2. AAPS PharmSciTech. 2023. PMID: 36949351
-
Continuous-Flow Production of Injectable Liposomes via a Microfluidic Approach.Materials (Basel). 2017 Dec 10;10(12):1411. doi: 10.3390/ma10121411. Materials (Basel). 2017. PMID: 29232873 Free PMC article.
-
Leveraging Numerical Simulation Technology to Advance Drug Preparation: A Comprehensive Review of Application Scenarios and Cases.Pharmaceutics. 2024 Oct 7;16(10):1304. doi: 10.3390/pharmaceutics16101304. Pharmaceutics. 2024. PMID: 39458634 Free PMC article. Review.
-
Neuroprotective Effect of Curcumin-Loaded RGD Peptide-PEGylated Nanoliposomes.Pharmaceutics. 2023 Nov 24;15(12):2665. doi: 10.3390/pharmaceutics15122665. Pharmaceutics. 2023. PMID: 38140006 Free PMC article.
-
Microfluidic approaches for producing lipid-based nanoparticles for drug delivery applications.Biophys Rev (Melville). 2023 Sep 18;4(3):031304. doi: 10.1063/5.0150345. eCollection 2023 Sep. Biophys Rev (Melville). 2023. PMID: 38505779 Free PMC article. Review.
References
-
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release. 2000;65(1-2):271–84. - PubMed
-
- O'Shaughnessy J. Liposomal anthracyclines for breast cancer: overview. The Oncologist. 2003;8(Suppl 2):1–2. - PubMed
-
- Patri AK, Majoros IJ, Baker JR. Dendritic polymer macromolecular carriers for drug delivery. Current Opinion in Chemical Biology. 2002 Aug;6(4):466–71. - PubMed
-
- Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Progress in Lipid Research. 2003 Nov;42(6):463–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

