Proteolytic processing of the prion protein in health and disease
- PMID: 23383379
- PMCID: PMC3560451
Proteolytic processing of the prion protein in health and disease
Abstract
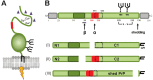
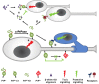
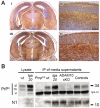
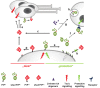
A variety of physiological functions, not only restricted to the nervous system, are discussed for the cellular prion protein (PrP(C)). A prominent, non-physiological property of PrPC is the conversion into its pathogenic isoform (PrP(Sc)) during fatal, transmissible, and neurodegenerative prion diseases. The prion protein is subject to posttranslational proteolytic processing and these cleavage events have been shown i) to regulate its physiological functions, ii) to produce biologically active fragments, and iii) to potentially influence the course of prion disease. Here, we give an overview on the proteolytic processing under physiological and pathological conditions and critically review what is currently known about the three main cleavage events of the prion protein, namely α-cleavage, β-cleavage, and ectodomain shedding. The biological relevance of resulting fragments as well as controversies regarding candidate proteases, with special emphasis on members of the A-disintegrin-and-metalloproteinase (ADAM) family, will be discussed. In addition, we make suggestions aimed at facilitating clarity and progress in this important research field. The better understanding of this issue will not only answer basic questions in prion biology but will likely impact research on other neurodegenerative diseases as well.
Keywords: ADAM10; ADAM17; Prion protein; ectodomain shedding; proteolytic processing; α-cleavage; β-cleavage.
Figures
Similar articles
-
Shedding light on prion disease.Prion. 2015;9(4):244-56. doi: 10.1080/19336896.2015.1065371. Prion. 2015. PMID: 26186508 Free PMC article.
-
Roles of endoproteolytic α-cleavage and shedding of the prion protein in neurodegeneration.FEBS J. 2013 Sep;280(18):4338-47. doi: 10.1111/febs.12196. Epub 2013 Mar 13. FEBS J. 2013. PMID: 23413979 Review.
-
Lack of a-disintegrin-and-metalloproteinase ADAM10 leads to intracellular accumulation and loss of shedding of the cellular prion protein in vivo.Mol Neurodegener. 2011 May 27;6:36. doi: 10.1186/1750-1326-6-36. Mol Neurodegener. 2011. PMID: 21619641 Free PMC article.
-
Endoproteolysis of cellular prion protein by plasmin hinders propagation of prions.Front Mol Neurosci. 2022 Sep 2;15:990136. doi: 10.3389/fnmol.2022.990136. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36117913 Free PMC article.
-
α-Cleavage of cellular prion protein.Prion. 2012 Nov-Dec;6(5):453-60. doi: 10.4161/pri.22511. Epub 2012 Oct 10. Prion. 2012. PMID: 23052041 Free PMC article. Review.
Cited by
-
Harnessing the Physiological Functions of Cellular Prion Protein in the Kidneys: Applications for Treating Renal Diseases.Biomolecules. 2021 May 22;11(6):784. doi: 10.3390/biom11060784. Biomolecules. 2021. PMID: 34067472 Free PMC article. Review.
-
Essential Components of Synthetic Infectious Prion Formation De Novo.Biomolecules. 2022 Nov 16;12(11):1694. doi: 10.3390/biom12111694. Biomolecules. 2022. PMID: 36421708 Free PMC article. Review.
-
Shedding light on prion disease.Prion. 2015;9(4):244-56. doi: 10.1080/19336896.2015.1065371. Prion. 2015. PMID: 26186508 Free PMC article.
-
Prion protein-Semisynthetic prion protein (PrP) variants with posttranslational modifications.J Pept Sci. 2019 Oct;25(10):e3216. doi: 10.1002/psc.3216. J Pept Sci. 2019. PMID: 31713950 Free PMC article. Review.
-
Prion protein modulates endothelial to mesenchyme-like transition in trabecular meshwork cells: Implications for primary open angle glaucoma.Sci Rep. 2019 Sep 11;9(1):13090. doi: 10.1038/s41598-019-49482-6. Sci Rep. 2019. PMID: 31511544 Free PMC article.
References
-
- Riek R, Hornemann S, Wider G, Glockshuber R, Wüthrich K. NMR characterization of the full-length recombinant murine prion protein, mPrP(23-231) FEBS Lett. 1997;413:282–288. - PubMed
-
- Brugger B, Graham C, Leibrecht I, Mombelli E, Jen A, Wieland F, Morris R. The membrane domains occupied by glycosylphosphatidylinositol-anchored prion protein and Thy-1 differ in lipid composition. J Biol Chem. 2004;279:7530–7536. - PubMed
-
- Taylor DR, Hooper NM. The prion protein and lipid rafts. Mol Membr Biol. 2006;23:89–99. - PubMed
-
- Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev. 2008;88:673–728. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
