Microcarrier-based expansion of adult murine side population stem cells
- PMID: 23383102
- PMCID: PMC3557248
- DOI: 10.1371/journal.pone.0055187
Microcarrier-based expansion of adult murine side population stem cells
Abstract
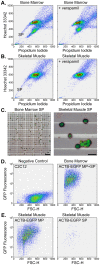
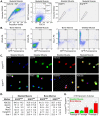
The lack of reliable methods to efficiently isolate and propagate stem cell populations is a significant obstacle to the advancement of cell-based therapies for human diseases. One isolation technique is based on efflux of the fluorophore Hoechst 33342. Using fluorescence-activated cell sorting (FACS), a sub-population containing adult stem cells has been identified in a multitude of tissues in every mammalian species examined. These rare cells are referred to as the 'side population' or SP due to a distinctive FACS profile that results from weak staining by Hoechst dye. Although the SP contains multi-potent cells capable of differentiating toward hematopoietic and mesenchymal lineages; there is currently no method to efficiently expand them. Here, we describe a spinner-flask culture system containing C2C12 myoblasts attached to spherical microcarriers that act to support the growth of non-adherent, post-natal murine skeletal muscle and bone marrow SP cells. Using FACS and hemocytometry, we show expansion of unfractionated EGFP⁺ SP cells over 6 wks. A significant number of these cells retain characteristics of freshly-isolated, unfractionated SP cells with respect to protein expression and dye efflux capacity. Expansion of the SP will permit further study of these heterogeneous cells and determine their therapeutic potential for regenerative and reparative therapies.
Conflict of interest statement
Figures


Similar articles
-
Growth of bone marrow and skeletal muscle side population stem cells in suspension culture.Methods Mol Biol. 2014;1210:51-61. doi: 10.1007/978-1-4939-1435-7_5. Methods Mol Biol. 2014. PMID: 25173160 Free PMC article.
-
Effective Detection of Hoechst Side Population Cells by Flow Cytometry.J Vis Exp. 2024 Aug 23;(210). doi: 10.3791/67012. J Vis Exp. 2024. PMID: 39248533
-
Sca+CD34- murine side population cells are highly enriched for primitive stem cells.Exp Hematol. 2003 Mar;31(3):244-50. doi: 10.1016/s0301-472x(02)01074-3. Exp Hematol. 2003. PMID: 12644022
-
Hematopoietic stem cell characterization and isolation.Methods Mol Biol. 2011;750:47-59. doi: 10.1007/978-1-61779-145-1_3. Methods Mol Biol. 2011. PMID: 21618082 Free PMC article. Review.
-
A side order of stem cells: the SP phenotype.Stem Cells. 2006 Jan;24(1):3-12. doi: 10.1634/stemcells.2005-0116. Stem Cells. 2006. PMID: 16449630 Review.
Cited by
-
Growth of bone marrow and skeletal muscle side population stem cells in suspension culture.Methods Mol Biol. 2014;1210:51-61. doi: 10.1007/978-1-4939-1435-7_5. Methods Mol Biol. 2014. PMID: 25173160 Free PMC article.
-
Shaped 3D microcarriers for adherent cell culture and analysis.Microsyst Nanoeng. 2018 Aug 13;4:21. doi: 10.1038/s41378-018-0020-7. eCollection 2018. Microsyst Nanoeng. 2018. PMID: 31057909 Free PMC article.
-
Muscle Atrophy Marker Expression Differs between Rotary Cell Culture System and Animal Studies.Biomed Res Int. 2019 Feb 17;2019:2042808. doi: 10.1155/2019/2042808. eCollection 2019. Biomed Res Int. 2019. PMID: 30906768 Free PMC article.
-
Stem cells in Osteoporosis: From Biology to New Therapeutic Approaches.Stem Cells Int. 2019 Jun 2;2019:1730978. doi: 10.1155/2019/1730978. eCollection 2019. Stem Cells Int. 2019. PMID: 31281368 Free PMC article. Review.
References
-
- Asakura A, Rudnicki MA (2002) Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol 30: 1339–1345. - PubMed
-
- Liadaki K, Kho AT, Sanoudou D, Schienda J, Flint A, et al. (2005) Side population cells isolated from different tissues share transcriptome signatures and express tissue-specific markers. Exp Cell Res 303: 360–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

