MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments
- PMID: 23383037
- PMCID: PMC3559346
- DOI: 10.1371/journal.pone.0055013
MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments
Abstract
Background: Oral and vaginal preparations of tenofovir as pre-exposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) infection have demonstrated variable efficacy in men and women prompting assessment of variation in drug concentration as an explanation. Knowledge of tenofovir concentration and its active form, tenofovir diphosphate, at the putative vaginal and rectal site of action and its relationship to concentrations at multiple other anatomic locations may provide key information for both interpreting PrEP study outcomes and planning future PrEP drug development.
Objective: MTN-001 was designed to directly compare oral to vaginal steady-state tenofovir pharmacokinetics in blood, vaginal tissue, and vaginal and rectal fluid in a paired cross-over design.
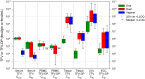
Methods and findings: We enrolled 144 HIV-uninfected women at 4 US and 3 African clinical research sites in an open label, 3-period crossover study of three different daily tenofovir regimens, each for 6 weeks (oral 300 mg tenofovir disoproxil fumarate, vaginal 1% tenofovir gel [40 mg], or both). Serum concentrations after vaginal dosing were 56-fold lower than after oral dosing (p<0.001). Vaginal tissue tenofovir diphosphate was quantifiable in ≥90% of women with vaginal dosing and only 19% of women with oral dosing. Vaginal tissue tenofovir diphosphate was ≥130-fold higher with vaginal compared to oral dosing (p<0.001). Rectal fluid tenofovir concentrations in vaginal dosing periods were higher than concentrations measured in the oral only dosing period (p<0.03).
Conclusions: Compared to oral dosing, vaginal dosing achieved much lower serum concentrations and much higher vaginal tissue concentrations. Even allowing for 100-fold concentration differences due to poor adherence or less frequent prescribed dosing, vaginal dosing of tenofovir should provide higher active site concentrations and theoretically greater PrEP efficacy than oral dosing; randomized topical dosing PrEP trials to the contrary indicates that factors beyond tenofovir's antiviral effect substantially influence PrEP efficacy.
Trial registration: ClinicalTrials.gov NCT00592124.
Conflict of interest statement
Figures

Similar articles
-
Pharmacokinetics and Pharmacodynamics of Tenofovir Reduced-Glycerin 1% Gel in the Rectal and Vaginal Compartments in Women: A Cross-Compartmental Study With Directly Observed Dosing.J Acquir Immune Defic Syndr. 2018 Jun 1;78(2):175-182. doi: 10.1097/QAI.0000000000001655. J Acquir Immune Defic Syndr. 2018. PMID: 29767639 Free PMC article. Clinical Trial.
-
RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate.AIDS Res Hum Retroviruses. 2012 Nov;28(11):1412-21. doi: 10.1089/aid.2012.0262. Epub 2012 Oct 9. AIDS Res Hum Retroviruses. 2012. PMID: 22943559 Free PMC article. Clinical Trial.
-
Adherence and acceptability in MTN 001: a randomized cross-over trial of daily oral and topical tenofovir for HIV prevention in women.AIDS Behav. 2013 Feb;17(2):737-47. doi: 10.1007/s10461-012-0333-8. AIDS Behav. 2013. PMID: 23065145 Free PMC article. Clinical Trial.
-
A drug evaluation of 1% tenofovir gel and tenofovir disoproxil fumarate tablets for the prevention of HIV infection.Expert Opin Investig Drugs. 2012 May;21(5):695-715. doi: 10.1517/13543784.2012.667072. Epub 2012 Mar 7. Expert Opin Investig Drugs. 2012. PMID: 22394224 Free PMC article. Review.
-
Considerations regarding antiretroviral chemoprophylaxis in MSM.Curr Opin HIV AIDS. 2012 Nov;7(6):549-56. doi: 10.1097/COH.0b013e3283582c71. Curr Opin HIV AIDS. 2012. PMID: 22918448 Review.
Cited by
-
Preclinical Considerations for Long-acting Delivery of Tenofovir Alafenamide from Subdermal Implants for HIV Pre-exposure Prophylaxis.Pharm Res. 2023 Jul;40(7):1657-1672. doi: 10.1007/s11095-022-03440-6. Epub 2022 Nov 23. Pharm Res. 2023. PMID: 36418671 Free PMC article.
-
Pharmacokinetics of antiretrovirals in mucosal tissue.Expert Opin Drug Metab Toxicol. 2015 Jun;11(6):893-905. doi: 10.1517/17425255.2015.1027682. Epub 2015 Mar 22. Expert Opin Drug Metab Toxicol. 2015. PMID: 25797064 Free PMC article. Review.
-
Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue.AIDS Res Hum Retroviruses. 2013 Nov;29(11):1443-50. doi: 10.1089/aid.2013.0044. Epub 2013 May 29. AIDS Res Hum Retroviruses. 2013. PMID: 23600365 Free PMC article. Clinical Trial.
-
Drug transporters in tissues and cells relevant to sexual transmission of HIV: Implications for drug delivery.J Control Release. 2015 Dec 10;219:681-696. doi: 10.1016/j.jconrel.2015.08.018. Epub 2015 Aug 13. J Control Release. 2015. PMID: 26278511 Free PMC article. Review.
-
Pharmacokinetics and Pharmacodynamics of Tenofovir Reduced-Glycerin 1% Gel in the Rectal and Vaginal Compartments in Women: A Cross-Compartmental Study With Directly Observed Dosing.J Acquir Immune Defic Syndr. 2018 Jun 1;78(2):175-182. doi: 10.1097/QAI.0000000000001655. J Acquir Immune Defic Syndr. 2018. PMID: 29767639 Free PMC article. Clinical Trial.
References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, et al... (2012) Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N Engl J Med. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

