Evidence of the presence of a functional Dot/Icm type IV-B secretion system in the fish bacterial pathogen Piscirickettsia salmonis
- PMID: 23383004
- PMCID: PMC3557282
- DOI: 10.1371/journal.pone.0054934
Evidence of the presence of a functional Dot/Icm type IV-B secretion system in the fish bacterial pathogen Piscirickettsia salmonis
Abstract
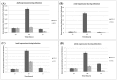
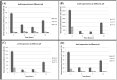
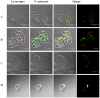
Piscirickettsia salmonis is a fish bacterial pathogen that has severely challenged the sustainability of the Chilean salmon industry since its appearance in 1989. As this Gram-negative bacterium has been poorly characterized, relevant aspects of its life cycle, virulence and pathogenesis must be identified in order to properly design prophylactic procedures. This report provides evidence of the functional presence in P. salmonis of four genes homologous to those described for Dot/Icm Type IV Secretion Systems. The Dot/Icm System, the major virulence mechanism of phylogenetically related pathogens Legionella pneumophila and Coxiella burnetii, is responsible for their intracellular survival and multiplication, conditions that may also apply to P. salmonis. Our results demonstrate that the four P. salmonis dot/icm homologues (dotB, dotA, icmK and icmE) are expressed both during in vitro tissue culture cells infection and growing in cell-free media, suggestive of their putative constitutive expression. Additionally, as it happens in other referential bacterial systems, temporal acidification of cell-free media results in over expression of all four P. salmonis genes, a well-known strategy by which SSTIV-containing bacteria inhibit phagosome-lysosome fusion to survive. These findings are very important to understand the virulence mechanisms of P. salmonis in order to design new prophylactic alternatives to control the disease.
Conflict of interest statement
Figures

Similar articles
-
In sílico identification and characterization of putative Dot/Icm secreted virulence effectors in the fish pathogen Piscirickettsia salmonis.Microb Pathog. 2016 Mar;92:11-18. doi: 10.1016/j.micpath.2015.12.002. Epub 2015 Dec 17. Microb Pathog. 2016. PMID: 26706346
-
Transcriptomic Changes of Piscirickettsia salmonis During Intracellular Growth in a Salmon Macrophage-Like Cell Line.Front Cell Infect Microbiol. 2020 Jan 9;9:426. doi: 10.3389/fcimb.2019.00426. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 31998656 Free PMC article.
-
In vitro expression of Sec-dependent pathway and type 4B secretion system in Piscirickettsia salmonis.Microb Pathog. 2017 Sep;110:586-593. doi: 10.1016/j.micpath.2017.08.003. Epub 2017 Aug 5. Microb Pathog. 2017. PMID: 28789875
-
Piscirickettsiosis and Piscirickettsia salmonis in fish: a review.J Fish Dis. 2014 Mar;37(3):163-88. doi: 10.1111/jfd.12211. Epub 2013 Nov 26. J Fish Dis. 2014. PMID: 24279295 Review.
-
The Icm/Dot type-IV secretion systems of Legionella pneumophila and Coxiella burnetii.FEMS Microbiol Rev. 2005 Jan;29(1):65-81. doi: 10.1016/j.femsre.2004.07.001. FEMS Microbiol Rev. 2005. PMID: 15652976 Review.
Cited by
-
Biofilm Produced In Vitro by Piscirickettsia salmonis Generates Differential Cytotoxicity Levels and Expression Patterns of Immune Genes in the Atlantic Salmon Cell Line SHK-1.Microorganisms. 2020 Oct 20;8(10):1609. doi: 10.3390/microorganisms8101609. Microorganisms. 2020. PMID: 33092013 Free PMC article.
-
Proximity ligation strategy for the genomic reconstruction of microbial communities associated with the ectoparasite Caligus rogercresseyi.Sci Rep. 2022 Jan 17;12(1):783. doi: 10.1038/s41598-021-04485-0. Sci Rep. 2022. PMID: 35039517 Free PMC article.
-
Intracellular Bacterial Infections: A Challenge for Developing Cellular Mediated Immunity Vaccines for Farmed Fish.Microorganisms. 2018 Apr 22;6(2):33. doi: 10.3390/microorganisms6020033. Microorganisms. 2018. PMID: 29690563 Free PMC article. Review.
-
RNA-Seq-Based Analysis of Cortisol-Induced Differential Gene Expression Associated with Piscirickettsia salmonis Infection in Rainbow Trout (Oncorhynchus mykiss) Myotubes.Animals (Basel). 2021 Aug 13;11(8):2399. doi: 10.3390/ani11082399. Animals (Basel). 2021. PMID: 34438856 Free PMC article.
-
Salmonid Rickettsial Septicemia (SRS) disease dynamics and Atlantic salmon immune response to Piscirickettsia salmonis LF-89 and EM-90 co-infection.Vet Res. 2024 Aug 16;55(1):102. doi: 10.1186/s13567-024-01356-0. Vet Res. 2024. PMID: 39152462 Free PMC article.
References
-
- Fryer JL, Lannan CN, Garcés HL, Larenas JL, Smith PA (1990) Isolation of Rickettsiales-like organism from diseased coho salmon (Oncorhynchuskisutch) in Chile. Fish Pathology 25: 107–114.
-
- Tobar JA, Jerez S, Caruffo M, Bravo C, Contreras F, et al. (2011) Oral vaccination of Atlantic salmon (Salmo salar) against salmonid rickettsialsepticaemia. Vaccine317: 83–92. - PubMed
-
- Bravo S, Campos M (1989) Síndrome del salmón Coho. Chile Pesquero 54: 47–48.
-
- Olsen AB, Evensen O, Speilberg I, Melby HP, Hastein T (1993) Nylaksesykdomforarsaketavrickettsie. NorskFiskeoppdrett 12: 40–J1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

