8-CPT-cAMP/all-trans retinoic acid targets t(11;17) acute promyelocytic leukemia through enhanced cell differentiation and PLZF/RARα degradation
- PMID: 23382200
- PMCID: PMC3587232
- DOI: 10.1073/pnas.1222863110
8-CPT-cAMP/all-trans retinoic acid targets t(11;17) acute promyelocytic leukemia through enhanced cell differentiation and PLZF/RARα degradation
Abstract
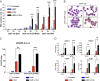
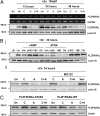
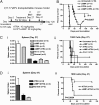
The refractoriness of acute promyelocytic leukemia (APL) with t(11;17)(q23;q21) to all-trans retinoic acid (ATRA)-based therapy concerns clinicians and intrigues basic researchers. By using a murine leukemic model carrying both promyelocytic leukemia zinc finger/retinoic acid receptor-α (PLZF/RARα) and RARα/PLZF fusion genes, we discovered that 8-chlorophenylthio adenosine-3', 5'-cyclic monophosphate (8-CPT-cAMP) enhances cellular differentiation and improves gene trans-activation by ATRA in leukemic blasts. Mechanistically, in combination with ATRA, 8-CPT-cAMP activates PKA, causing phosphorylation of PLZF/RARα at Ser765 and resulting in increased dissociation of the silencing mediator for retinoic acid and thyroid hormone receptors/nuclear receptor corepressor from PLZF/RARα. This process results in changes of local chromatin and transcriptional reactivation of the retinoic acid pathway in leukemic cells. Meanwhile, 8-CPT-cAMP also potentiated ATRA-induced degradation of PLZF/RARα through its Ser765 phosphorylation. In vivo treatment of the t(11;17) APL mouse model demonstrated that 8-CPT-cAMP could significantly improve the therapeutic effect of ATRA by targeting a leukemia-initiating cell activity. This combined therapy, which induces enhanced differentiation and oncoprotein degradation, may benefit t(11;17) APL patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia.Blood. 1998 Apr 15;91(8):2634-42. Blood. 1998. PMID: 9531570
-
Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARalpha and PLZF-RARalpha oncoproteins.Proc Natl Acad Sci U S A. 2000 Aug 29;97(18):10173-8. doi: 10.1073/pnas.180290497. Proc Natl Acad Sci U S A. 2000. PMID: 10954752 Free PMC article.
-
Retinoic acid, but not arsenic trioxide, degrades the PLZF/RARalpha fusion protein, without inducing terminal differentiation or apoptosis, in a RA-therapy resistant t(11;17)(q23;q21) APL patient.Oncogene. 1999 Jan 28;18(4):1113-8. doi: 10.1038/sj.onc.1202414. Oncogene. 1999. PMID: 10023688
-
Acute promyelocytic leukemia with a PLZF-RARalpha fusion protein.Semin Hematol. 2001 Jan;38(1):37-41. doi: 10.1016/s0037-1963(01)90004-6. Semin Hematol. 2001. PMID: 11172538 Review.
-
Understanding the molecular pathogenesis of acute promyelocytic leukemia.Best Pract Res Clin Haematol. 2014 Mar;27(1):3-9. doi: 10.1016/j.beha.2014.04.006. Epub 2014 Apr 13. Best Pract Res Clin Haematol. 2014. PMID: 24907012 Review.
Cited by
-
Hyperthermia promotes degradation of the acute promyelocytic leukemia driver oncoprotein ZBTB16/RARα.Acta Pharmacol Sin. 2023 Apr;44(4):822-831. doi: 10.1038/s41401-022-01001-6. Epub 2022 Oct 10. Acta Pharmacol Sin. 2023. PMID: 36216898 Free PMC article.
-
Treatment with Cyclic AMP Activators Reduces Glioblastoma Growth and Invasion as Assessed by Two-Photon Microscopy.Cells. 2021 Mar 4;10(3):556. doi: 10.3390/cells10030556. Cells. 2021. PMID: 33806549 Free PMC article.
-
Function of PML-RARA in Acute Promyelocytic Leukemia.Adv Exp Med Biol. 2024;1459:321-339. doi: 10.1007/978-3-031-62731-6_14. Adv Exp Med Biol. 2024. PMID: 39017850 Review.
-
Synergy from gene expression and network mining (SynGeNet) method predicts synergistic drug combinations for diverse melanoma genomic subtypes.NPJ Syst Biol Appl. 2019 Feb 26;5:6. doi: 10.1038/s41540-019-0085-4. eCollection 2019. NPJ Syst Biol Appl. 2019. PMID: 30820351 Free PMC article.
-
Current views on the genetic landscape and management of variant acute promyelocytic leukemia.Biomark Res. 2021 May 6;9(1):33. doi: 10.1186/s40364-021-00284-x. Biomark Res. 2021. PMID: 33957999 Free PMC article. Review.
References
-
- Zelent A, Guidez F, Melnick A, Waxman S, Licht JD. Translocations of the RARalpha gene in acute promyelocytic leukemia. Oncogene. 2001;20(49):7186–7203. - PubMed
-
- Wang ZY, Chen Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood. 2008;111(5):2505–2515. - PubMed
-
- Licht JD, et al. Clinical and molecular characterization of a rare syndrome of acute promyelocytic leukemia associated with translocation (11;17) Blood. 1995;85(4):1083–1094. - PubMed
-
- Jansen JH, et al. Complete remission of t(11;17) positive acute promyelocytic leukemia induced by all-trans retinoic acid and granulocyte colony-stimulating factor. Blood. 1999;94(1):39–45. - PubMed
-
- Petti MC, et al. Complete remission through blast cell differentiation in PLZF/RARalpha-positive acute promyelocytic leukemia: in vitro and in vivo studies. Blood. 2002;100(3):1065–1067. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

