Quantitative analysis of persister fractions suggests different mechanisms of formation among environmental isolates of E. coli
- PMID: 23379956
- PMCID: PMC3682893
- DOI: 10.1186/1471-2180-13-25
Quantitative analysis of persister fractions suggests different mechanisms of formation among environmental isolates of E. coli
Abstract
Background: Bacterial persistence describes a phenomenon wherein a small subpopulation of cells is able to survive a challenge with high doses of an antibiotic (or other stressor) better than the majority of the population. Previous work has shown that cells that are in a dormant or slow-growing state are persistent to antibiotic treatment and that populations with higher fractions of dormant cells exhibit higher levels of persistence. These data suggest that a major determinant of the fraction of persisters within a population is the rate at which cells enter and exit from dormancy. However, it is not known whether there are physiological changes in addition to dormancy that influence persistence. Here, we use quantitative measurements of persister fractions in a set of environmental isolates of E. coli together with a mathematical model of persister formation to test whether a single general physiological change, such as cell dormancy, can explain the differences in persister phenotypes observed in different strains.
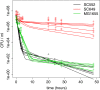
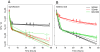
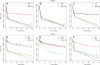
Results: If a single physiological change (e.g. cell dormancy) underlies most persister phenotypes, then strains should exhibit characteristic fractions of persister cells: some strains will consistently have high fractions of persisters (dormant cells), whereas others will have low fractions. Although we found substantial variation in the fraction of persisters between different environmental isolates of E. coli, these fractions were not correlated across antibiotics. Some strains exhibited high persister fractions in one antibiotic, but low persister fractions in a second antibiotic. Surprisingly, no correlation in persister fractions was observed between any two drugs, even for antibiotics with nearly identical modes of action (ciprofloxacin and nalidixic acid).
Conclusions: These data support the hypothesis that there is no single physiological change that determines the persistence level in a population of cells. Instead, the fraction of cells that survive antibiotic treatment (persist) depends critically on the specific antibiotic that is used, suggesting that physiological changes in addition to dormancy can underlie persister phenotypes.
Figures
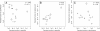


Similar articles
-
Prophages and Growth Dynamics Confound Experimental Results with Antibiotic-Tolerant Persister Cells.mBio. 2017 Dec 12;8(6):e01964-17. doi: 10.1128/mBio.01964-17. mBio. 2017. PMID: 29233898 Free PMC article.
-
Pseudomonas syringae pv. phaseolicola Uses Distinct Modes of Stationary-Phase Persistence To Survive Bacteriocin and Streptomycin Treatments.mBio. 2021 Apr 13;12(2):e00161-21. doi: 10.1128/mBio.00161-21. mBio. 2021. PMID: 33849974 Free PMC article.
-
Persister Escherichia coli Cells Have a Lower Intracellular pH than Susceptible Cells but Maintain Their pH in Response to Antibiotic Treatment.mBio. 2021 Aug 31;12(4):e0090921. doi: 10.1128/mBio.00909-21. Epub 2021 Jul 20. mBio. 2021. PMID: 34281389 Free PMC article.
-
Bacterial persister cell formation and dormancy.Appl Environ Microbiol. 2013 Dec;79(23):7116-21. doi: 10.1128/AEM.02636-13. Epub 2013 Sep 13. Appl Environ Microbiol. 2013. PMID: 24038684 Free PMC article. Review.
-
A Historical Perspective on Bacterial Persistence.Methods Mol Biol. 2016;1333:3-13. doi: 10.1007/978-1-4939-2854-5_1. Methods Mol Biol. 2016. PMID: 26468095 Review.
Cited by
-
Antibiotic Tolerance Indicative of Persistence Is Pervasive among Clinical Streptococcus pneumoniae Isolates and Shows Strong Condition Dependence.Microbiol Spectr. 2022 Dec 21;10(6):e0270122. doi: 10.1128/spectrum.02701-22. Epub 2022 Nov 14. Microbiol Spectr. 2022. PMID: 36374111 Free PMC article.
-
Relationship between Tolerance and Persistence Mechanisms in Acinetobacter baumannii Strains with AbkAB Toxin-Antitoxin System.Antimicrob Agents Chemother. 2018 Apr 26;62(5):e00250-18. doi: 10.1128/AAC.00250-18. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29463538 Free PMC article.
-
UNRAVELING CRP/cAMP-MEDIATED METABOLIC REGULATION IN ESCHERICHIA COLI PERSISTER CELLS.bioRxiv [Preprint]. 2024 Jun 10:2024.06.10.598332. doi: 10.1101/2024.06.10.598332. bioRxiv. 2024. PMID: 38915711 Free PMC article. Preprint.
-
Contribution of the Chromosomal ccdAB Operon to Bacterial Drug Tolerance.J Bacteriol. 2017 Sep 5;199(19):e00397-17. doi: 10.1128/JB.00397-17. Print 2017 Oct 1. J Bacteriol. 2017. PMID: 28674066 Free PMC article.
-
Formation of Escherichia coli O157:H7 Persister Cells in the Lettuce Phyllosphere and Application of Differential Equation Models To Predict Their Prevalence on Lettuce Plants in the Field.Appl Environ Microbiol. 2020 Jan 7;86(2):e01602-19. doi: 10.1128/AEM.01602-19. Print 2020 Jan 7. Appl Environ Microbiol. 2020. PMID: 31704677 Free PMC article.
References
-
- Bigger JW. Treatment of staphylococcal infections with penicillin - by intermittent sterilisation. Lancet. 1944;2:497–500.
-
- Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. The rate of killing of escherichia-coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial-growth. J Gen Microbiol. 1986;132:1297–1304. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

