The nicotine-mediated decline in l-dopa-induced dyskinesias is associated with a decrease in striatal dopamine release
- PMID: 23373725
- PMCID: PMC3778041
- DOI: 10.1111/jnc.12179
The nicotine-mediated decline in l-dopa-induced dyskinesias is associated with a decrease in striatal dopamine release
Abstract
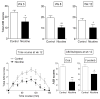
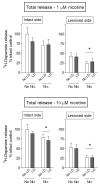
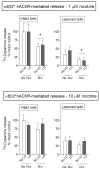
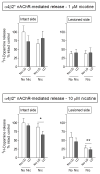
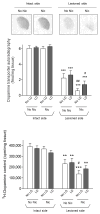
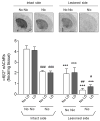
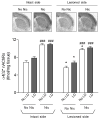
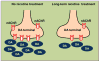
l-dopa-induced dyskinesias (LIDs) are a side effect of Parkinson's disease therapy that is thought to arise, at least in part, because of excessive dopaminergic activity. Thus, drugs that regulate dopaminergic tone may provide an approach to manage LIDs. Our previous studies showed that nicotine treatment reduced LIDs in Parkinsonian animal models. This study investigates whether nicotine may exert its beneficial effects by modulating pre-synaptic dopaminergic function. Rats were unilaterally lesioned by injection of 6-hydroxydopamine (6-OHDA) (2 × 3 ug per site) into the medial forebrain bundle to yield moderate Parkinsonism. They were then implanted with minipumps containing vehicle or nicotine (2.0 mg/kg/d) and rendered dyskinetic with l-dopa (8 mg/kg plus 15 mg/kg benserazide). Lesioning alone decreased the striatal dopamine transporter, nicotinic receptor (nAChR) levels, and nAChR-mediated (3)H-dopamine release, consistent with previous results. Nicotine administration reduced l-dopa-induced abnormal involuntary movements throughout the course of the study (4 months). Nicotine treatment led to declines in the striatal dopamine transporter, α6β2* nAChRs and various components of α6β2* and α4β2* nAChR-mediated release. l-dopa treatment had no effect. These data suggest that nicotine may improve LIDs in Parkinsonian animal models by dampening striatal dopaminergic activity.
Keywords: LIDs; dopamine; nicotine; nicotinic receptors; nigrostriatal lesion.
© 2013 International Society for Neurochemistry.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Nicotine-mediated improvement in L-dopa-induced dyskinesias in MPTP-lesioned monkeys is dependent on dopamine nerve terminal function.Neurobiol Dis. 2013 Feb;50:30-41. doi: 10.1016/j.nbd.2012.09.006. Epub 2012 Sep 23. Neurobiol Dis. 2013. PMID: 23009753 Free PMC article.
-
α4β2 Nicotinic receptors play a role in the nAChR-mediated decline in L-dopa-induced dyskinesias in parkinsonian rats.Neuropharmacology. 2013 Aug;71:191-203. doi: 10.1016/j.neuropharm.2013.03.038. Epub 2013 Apr 12. Neuropharmacology. 2013. PMID: 23583932 Free PMC article.
-
Role for α6 nicotinic receptors in l-dopa-induced dyskinesias in parkinsonian mice.Neuropharmacology. 2012 Sep;63(3):450-9. doi: 10.1016/j.neuropharm.2012.04.029. Epub 2012 May 3. Neuropharmacology. 2012. PMID: 22579614 Free PMC article.
-
Role for the nicotinic cholinergic system in movement disorders; therapeutic implications.Pharmacol Ther. 2014 Oct;144(1):50-9. doi: 10.1016/j.pharmthera.2014.05.004. Epub 2014 May 14. Pharmacol Ther. 2014. PMID: 24836728 Free PMC article. Review.
-
mGlu5, Dopamine D2 and Adenosine A2A Receptors in L-DOPA-induced Dyskinesias.Curr Neuropharmacol. 2016;14(5):481-93. doi: 10.2174/1570159x14666151201185652. Curr Neuropharmacol. 2016. PMID: 26639458 Free PMC article. Review.
Cited by
-
α6β2*-subtype nicotinic acetylcholine receptors are more sensitive than α4β2*-subtype receptors to regulation by chronic nicotine administration.J Neurochem. 2014 Jul;130(2):185-98. doi: 10.1111/jnc.12721. Epub 2014 Apr 19. J Neurochem. 2014. PMID: 24661093 Free PMC article.
-
Alpha7 nicotinic receptors as therapeutic targets for Parkinson's disease.Biochem Pharmacol. 2015 Oct 15;97(4):399-407. doi: 10.1016/j.bcp.2015.06.014. Epub 2015 Jun 18. Biochem Pharmacol. 2015. PMID: 26093062 Free PMC article. Review.
-
Receptor Ligands as Helping Hands to L-DOPA in the Treatment of Parkinson's Disease.Biomolecules. 2019 Apr 9;9(4):142. doi: 10.3390/biom9040142. Biomolecules. 2019. PMID: 30970612 Free PMC article. Review.
-
Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson's disease.J Pharmacol Exp Ther. 2013 Oct;347(1):225-34. doi: 10.1124/jpet.113.207639. Epub 2013 Jul 31. J Pharmacol Exp Ther. 2013. PMID: 23902940 Free PMC article.
-
Evidence for a role for α6(∗) nAChRs in l-dopa-induced dyskinesias using Parkinsonian α6(∗) nAChR gain-of-function mice.Neuroscience. 2015 Jun 4;295:187-97. doi: 10.1016/j.neuroscience.2015.03.040. Epub 2015 Mar 24. Neuroscience. 2015. PMID: 25813704 Free PMC article.
References
-
- Artymyshyn R, Smith A, Wolfe BB. The use of 3H standards in 125I autoradiography. J Neurosci Methods. 1990;32:185–192. - PubMed
-
- Berthet A, Bezard E. Dopamine receptors and L-dopa-induced dyskinesia. Parkinsonism Relat Disord. 2009;15(Suppl 4):S8–12. - PubMed
-
- Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci. 2001;2:577–588. - PubMed
-
- Bordia T, Campos C, Huang L, Quik M. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2008;327:239–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

