Nanoparticle mediated P-glycoprotein silencing for improved drug delivery across the blood-brain barrier: a siRNA-chitosan approach
- PMID: 23372682
- PMCID: PMC3553124
- DOI: 10.1371/journal.pone.0054182
Nanoparticle mediated P-glycoprotein silencing for improved drug delivery across the blood-brain barrier: a siRNA-chitosan approach
Abstract
The blood-brain barrier (BBB), composed of tightly organized endothelial cells, limits the availability of drugs to therapeutic targets in the central nervous system. The barrier is maintained by membrane bound efflux pumps efficiently transporting specific xenobiotics back into the blood. The efflux pump P-glycoprotein (P-gp), expressed at high levels in brain endothelial cells, has several drug substrates. Consequently, siRNA mediated silencing of the P-gp gene is one possible strategy how to improve the delivery of drugs to the brain. Herein, we investigated the potential of siRNA-chitosan nanoparticles in silencing P-gp in a BBB model. We show that the transfection of rat brain endothelial cells mediated effective knockdown of P-gp with subsequent decrease in P-gp substrate efflux. This resulted in increased cellular delivery and efficacy of the model drug doxorubicin.
Conflict of interest statement
Figures
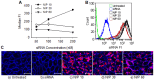
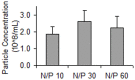
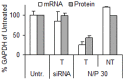



Similar articles
-
Validation of Thiosemicarbazone Compounds as P-Glycoprotein Inhibitors in Human Primary Brain-Blood Barrier and Glioblastoma Stem Cells.Mol Pharm. 2019 Aug 5;16(8):3361-3373. doi: 10.1021/acs.molpharmaceut.9b00018. Epub 2019 Jul 2. Mol Pharm. 2019. PMID: 31265310
-
An electrically tight in vitro blood-brain barrier model displays net brain-to-blood efflux of substrates for the ABC transporters, P-gp, Bcrp and Mrp-1.AAPS J. 2014 Sep;16(5):1046-55. doi: 10.1208/s12248-014-9628-1. Epub 2014 Jun 17. AAPS J. 2014. PMID: 24934296 Free PMC article.
-
Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes.Drug Deliv Transl Res. 2017 Aug;7(4):497-506. doi: 10.1007/s13346-017-0368-5. Drug Deliv Transl Res. 2017. PMID: 28315051
-
Regulation of P-Glycoprotein in the Brain.Int J Mol Sci. 2022 Nov 24;23(23):14667. doi: 10.3390/ijms232314667. Int J Mol Sci. 2022. PMID: 36498995 Free PMC article. Review.
-
Potential Regulation Mechanisms of P-gp in the Blood-Brain Barrier in Hypoxia.Curr Pharm Des. 2019;25(10):1041-1051. doi: 10.2174/1381612825666190610140153. Curr Pharm Des. 2019. PMID: 31187705 Review.
Cited by
-
Nanocarrier-based therapies for CNS tumors.CNS Oncol. 2014 Mar;3(2):115-22. doi: 10.2217/cns.14.2. CNS Oncol. 2014. PMID: 25055017 Free PMC article. Review.
-
Mad2 checkpoint gene silencing using epidermal growth factor receptor-targeted chitosan nanoparticles in non-small cell lung cancer model.Mol Pharm. 2014 Oct 6;11(10):3515-27. doi: 10.1021/mp5002894. Epub 2014 Sep 26. Mol Pharm. 2014. PMID: 25256346 Free PMC article.
-
Advanced gene nanocarriers/scaffolds in nonviral-mediated delivery system for tissue regeneration and repair.J Nanobiotechnology. 2024 Jun 26;22(1):376. doi: 10.1186/s12951-024-02580-8. J Nanobiotechnology. 2024. PMID: 38926780 Free PMC article. Review.
-
Grafting chitosan with polyethylenimine in an ionic liquid for efficient gene delivery.PLoS One. 2015 Apr 13;10(4):e0121817. doi: 10.1371/journal.pone.0121817. eCollection 2015. PLoS One. 2015. PMID: 25875475 Free PMC article.
-
Chitosan siRNA Nanoparticles Produce Significant Non-Toxic Functional Gene Silencing in Kidney Cortices.Polymers (Basel). 2024 Sep 9;16(17):2547. doi: 10.3390/polym16172547. Polymers (Basel). 2024. PMID: 39274180 Free PMC article.
References
-
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, et al. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498. - PubMed
-
- Khoury M, Louis-Plence P, Escriou V, Noel D, Largeau C, et al. (2006) Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum 54: 1867–1877. - PubMed
-
- Malmo J, Sørgård H, Vårum KM, Strand SP (2012) siRNA delivery with chitosan nanoparticles: Molecular properties favoring efficient gene silencing. J Control Release 158: 261–268. - PubMed
-
- Grayson AR, Doody A, Putnam D (2006) Biophysical and Structural Characterization of Polyethylenimine-Mediated siRNA Delivery in Vitro. Pharmaceut Res 23: 1868–1876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

