Accumulation of intraneuronal β-amyloid 42 peptides is associated with early changes in microtubule-associated protein 2 in neurites and synapses
- PMID: 23372648
- PMCID: PMC3553177
- DOI: 10.1371/journal.pone.0051965
Accumulation of intraneuronal β-amyloid 42 peptides is associated with early changes in microtubule-associated protein 2 in neurites and synapses
Abstract
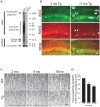
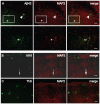
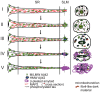
Pathologic aggregation of β-amyloid (Aβ) peptide and the axonal microtubule-associated protein tau protein are hallmarks of Alzheimer's disease (AD). Evidence supports that Aβ peptide accumulation precedes microtubule-related pathology, although the link between Aβ and tau remains unclear. We previously provided evidence for early co-localization of Aβ42 peptides and hyperphosphorylated tau within postsynaptic terminals of CA1 dendrites in the hippocampus of AD transgenic mice. Here, we explore the relation between Aβ peptide accumulation and the dendritic, microtubule-associated protein 2 (MAP2) in the well-characterized amyloid precursor protein Swedish mutant transgenic mouse (Tg2576). We provide evidence that localized intraneuronal accumulation of Aβ42 peptides is spatially associated with reductions of MAP2 in dendrites and postsynaptic compartments of Tg2576 mice at early ages. Our data support that reduction in MAP2 begins at sites of Aβ42 monomer and low molecular weight oligomer (M/LMW) peptide accumulation. Cumulative evidence suggests that accumulation of M/LMW Aβ42 peptides occurs early, before high molecular weight oligomerization and plaque formation. Since synaptic alteration is the best pathologic correlate of cognitive dysfunction in AD, the spatial association of M/LMW Aβ peptide accumulation with pathology of MAP2 within neuronal processes and synaptic compartments early in the disease process reinforces the importance of intraneuronal Aβ accumulation in AD pathogenesis.
Conflict of interest statement
Figures

Similar articles
-
High-resolution 3D reconstruction reveals intra-synaptic amyloid fibrils.Am J Pathol. 2011 Nov;179(5):2551-8. doi: 10.1016/j.ajpath.2011.07.045. Epub 2011 Sep 15. Am J Pathol. 2011. PMID: 21925470 Free PMC article.
-
Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain.J Neurosci. 2004 Apr 7;24(14):3592-9. doi: 10.1523/JNEUROSCI.5167-03.2004. J Neurosci. 2004. PMID: 15071107 Free PMC article.
-
Co-occurrence of Alzheimer's disease ß-amyloid and τ pathologies at synapses.Neurobiol Aging. 2010 Jul;31(7):1145-52. doi: 10.1016/j.neurobiolaging.2008.07.021. Epub 2008 Sep 3. Neurobiol Aging. 2010. PMID: 18771816 Free PMC article.
-
The inside-out amyloid hypothesis and synapse pathology in Alzheimer's disease.Neurodegener Dis. 2014;13(2-3):142-6. doi: 10.1159/000354776. Epub 2013 Sep 24. Neurodegener Dis. 2014. PMID: 24080821 Review.
-
Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review.
Cited by
-
Impaired retrograde transport of axonal autophagosomes contributes to autophagic stress in Alzheimer's disease neurons.Elife. 2017 Jan 13;6:e21776. doi: 10.7554/eLife.21776. Elife. 2017. PMID: 28085665 Free PMC article.
-
Dementia of the eye: the role of amyloid beta in retinal degeneration.Eye (Lond). 2015 Aug;29(8):1013-26. doi: 10.1038/eye.2015.100. Epub 2015 Jun 19. Eye (Lond). 2015. PMID: 26088679 Free PMC article. Review.
-
Amyloid β oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis.Acta Neuropathol. 2015 Feb;129(2):183-206. doi: 10.1007/s00401-015-1386-3. Epub 2015 Jan 22. Acta Neuropathol. 2015. PMID: 25604547 Free PMC article. Review.
-
A novel structure associated with aging is augmented in the DPP6-KO mouse brain.Acta Neuropathol Commun. 2020 Nov 23;8(1):197. doi: 10.1186/s40478-020-01065-7. Acta Neuropathol Commun. 2020. PMID: 33225987 Free PMC article.
-
Early Dendritic Dystrophy in Human Brains With Primary Age-Related Tauopathy.Front Aging Neurosci. 2020 Dec 7;12:596894. doi: 10.3389/fnagi.2020.596894. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33364934 Free PMC article.
References
-
- Walsh DM, Selkoe DJ (2004) Deciphering the Molecular Basis of Memory Failure in Alzheimer's Disease. Neuron 44: 181–193. - PubMed
-
- Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, et al. (2002) Altered Metabolism of the Amyloid β Precursor Protein Is Associated with Mitochondrial Dysfunction in Down's Syndrome. Neuron 33: 677–688. - PubMed
-
- Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, et al. (2004) Aβ localization in abnormal endosomes: association with earliest Aβ elevations in AD and Down syndrome. Neurobiol Aging 25: 1263–1272. - PubMed
-
- D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DHS (2001) Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology 38: 120–134. - PubMed
-
- Echeverria V, Cuello AC (2002) Intracellular A-beta amyloid, a sign for worse things to come? Mol Neurobiol 26: 299–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

