Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling
- PMID: 23363784
- PMCID: PMC3615161
- DOI: 10.18632/aging.100521
Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling
Abstract
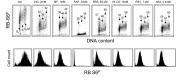
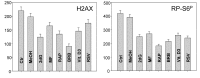
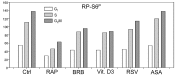
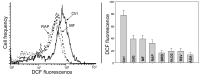
Two different mechanisms are considered to be the primary cause of aging. Cumulative DNA damage caused by reactive oxygen species (ROS), the by-products of oxidative phosphorylation, is one of these mechanisms (ROS concept). Constitutive stimulation of mitogen- and nutrient-sensing mTOR/S6 signaling is the second mechanism (TOR concept). The flow- and laser scanning- cytometric methods were developed to measure the level of the constitutive DNA damage/ROS- as well as of mTOR/S6- signaling in individual cells. Specifically, persistent activation of ATM and expression of γH2AX in untreated cells appears to report constitutive DNA damage induced by endogenous ROS. The level of phosphorylation of Ser235/236-ribosomal protein (RP), of Ser2448-mTOR and of Ser65-4EBP1, informs on constitutive signaling along the mTOR/S6 pathway. Potential gero-suppressive agents rapamycin, metformin, 2-deoxyglucose, berberine, resveratrol, vitamin D3 and aspirin, all decreased the level of constitutive DNA damage signaling as seen by the reduced expression of γH2AX in proliferating A549, TK6, WI-38 cells and in mitogenically stimulated human lymphocytes. They all also decreased the level of intracellular ROS and mitochondrial trans-membrane potential ΔΨm, the marker of mitochondrial energizing as well as reduced phosphorylation of mTOR, RP-S6 and 4EBP1. The most effective was rapamycin. Although the primary target of each on these agents may be different the data are consistent with the downstream mechanism in which the decline in mTOR/S6K signaling and translation rate is coupled with a decrease in oxidative phosphorylation, (revealed by ΔΨm) that leads to reduction of ROS and oxidative DNA damage. The decreased rate of translation induced by these agents may slow down cells hypertrophy and alleviate other features of cell aging/senescence. Reduction of oxidative DNA damage may lower predisposition to neoplastic transformation which otherwise may result from errors in repair of DNA sites coding for oncogenes or tumor suppressor genes. The data suggest that combined assessment of constitutive γH2AX expression, mitochondrial activity (ROS, ΔΨm) and mTOR signaling provides an adequate gamut of cell responses to evaluate effectiveness of gero-suppressive agents.
Conflict of interest statement
The authors of this manuscript have no conflict of interests to declare.
Figures




Similar articles
-
In search of antiaging modalities: evaluation of mTOR- and ROS/DNA damage-signaling by cytometry.Cytometry A. 2014 May;85(5):386-99. doi: 10.1002/cyto.a.22452. Epub 2014 Feb 22. Cytometry A. 2014. PMID: 24677687 Free PMC article. Review.
-
Berberine suppresses gero-conversion from cell cycle arrest to senescence.Aging (Albany NY). 2013 Aug;5(8):623-36. doi: 10.18632/aging.100593. Aging (Albany NY). 2013. PMID: 23974852 Free PMC article.
-
Genome protective effect of metformin as revealed by reduced level of constitutive DNA damage signaling.Aging (Albany NY). 2011 Oct;3(10):1028-38. doi: 10.18632/aging.100397. Aging (Albany NY). 2011. PMID: 22067284 Free PMC article.
-
Attenuation of constitutive DNA damage signaling by 1,25-dihydroxyvitamin D3.Aging (Albany NY). 2012 Apr;4(4):270-8. doi: 10.18632/aging.100450. Aging (Albany NY). 2012. PMID: 22498490 Free PMC article.
-
Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants.Cell Cycle. 2006 Sep;5(17):1940-5. doi: 10.4161/cc.5.17.3191. Epub 2006 Sep 1. Cell Cycle. 2006. PMID: 16940754 Free PMC article. Review.
Cited by
-
M(o)TOR of aging: MTOR as a universal molecular hypothalamus.Aging (Albany NY). 2013 Jul;5(7):490-4. doi: 10.18632/aging.100580. Aging (Albany NY). 2013. PMID: 23872658 Free PMC article.
-
Dietary Resveratrol Does Not Affect Life Span, Body Composition, Stress Response, and Longevity-Related Gene Expression in Drosophila melanogaster.Int J Mol Sci. 2018 Jan 11;19(1):223. doi: 10.3390/ijms19010223. Int J Mol Sci. 2018. PMID: 29324667 Free PMC article.
-
Chemical inhibition of acetyl-CoA carboxylase suppresses self-renewal growth of cancer stem cells.Oncotarget. 2014 Sep 30;5(18):8306-16. doi: 10.18632/oncotarget.2059. Oncotarget. 2014. PMID: 25246709 Free PMC article.
-
Comparison of rapamycin schedules in mice on high-fat diet.Cell Cycle. 2014;13(21):3350-6. doi: 10.4161/15384101.2014.970491. Cell Cycle. 2014. PMID: 25485580 Free PMC article.
-
From rapalogs to anti-aging formula.Oncotarget. 2017 May 30;8(22):35492-35507. doi: 10.18632/oncotarget.18033. Oncotarget. 2017. PMID: 28548953 Free PMC article. Review.
References
-
- Barzilai A, Yamamoto K. DNA damage responses to oxidative stress. DNA repair (Amst) 2004;3:1109–1115. - PubMed
-
- Moller P, Loft S. Interventions with antioxidants and nutrients in relation to oxidative DNA damage and repair. Mutat Res. 2004;551:79–89. - PubMed
-
- Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:13300–13305. - PubMed
-
- Karanjawala ZE, Lieber MR. DNA damage and aging. Mech Ageing Dev. 2004;125:405–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

