H2O2 regulates lung epithelial sodium channel (ENaC) via ubiquitin-like protein Nedd8
- PMID: 23362276
- PMCID: PMC3605632
- DOI: 10.1074/jbc.M112.389536
H2O2 regulates lung epithelial sodium channel (ENaC) via ubiquitin-like protein Nedd8
Abstract
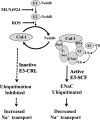
Redundancies in both the ubiquitin and epithelial sodium transport pathways allude to their importance of proteolytic degradation and ion transport in maintaining normal cell function. The classical pathway implicated in ubiquitination of the epithelial sodium channel (ENaC) involves Nedd4-2 regulation of sodium channel subunit expression and has been studied extensively studied. However, less attention has been given to the role of the ubiquitin-like protein Nedd8. Here we show that Nedd8 plays an important role in the ubiquitination of ENaC in alveolar epithelial cells. We report that the Nedd8 pathway is redox-sensitive and that under oxidizing conditions Nedd8 conjugation to Cullin-1 is attenuated, resulting in greater surface expression of α-ENaC. This observation was confirmed in our electrophysiology studies in which we inhibited Nedd8-activating enzyme using MLN4924 (a specific Nedd8-activating enzyme inhibitor) and observed a marked increase in ENaC activity (measured as the product of the number of channels (N) and the open probability (Po) of a channel). These results suggest that ubiquitination of lung ENaC is redox-sensitive and may have significant implications for our understanding of the role of ENaC in pulmonary conditions where oxidative stress occurs, such as pulmonary edema and acute lung injury.
Figures







Similar articles
-
Hypoxia-induced inhibition of epithelial Na(+) channels in the lung. Role of Nedd4-2 and the ubiquitin-proteasome pathway.Am J Respir Cell Mol Biol. 2014 Mar;50(3):526-37. doi: 10.1165/rcmb.2012-0518OC. Am J Respir Cell Mol Biol. 2014. PMID: 24093724
-
NEDD8 overexpression results in neddylation of ubiquitin substrates by the ubiquitin pathway.J Mol Biol. 2012 Aug 3;421(1):27-9. doi: 10.1016/j.jmb.2012.05.013. Epub 2012 May 15. J Mol Biol. 2012. PMID: 22608973
-
Nedd8-Activating Enzyme Is a Druggable Host Dependency Factor of Human and Mouse Cytomegalovirus.Viruses. 2021 Aug 14;13(8):1610. doi: 10.3390/v13081610. Viruses. 2021. PMID: 34452475 Free PMC article.
-
Regulation of epithelial sodium channel trafficking by ubiquitination.Proc Am Thorac Soc. 2010 Feb;7(1):54-64. doi: 10.1513/pats.200909-096JS. Proc Am Thorac Soc. 2010. PMID: 20160149 Free PMC article. Review.
-
Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination.Kidney Int. 2000 Mar;57(3):809-15. doi: 10.1046/j.1523-1755.2000.00919.x. Kidney Int. 2000. PMID: 10720933 Review.
Cited by
-
Acute effects of cigarette smoke extract on alveolar epithelial sodium channel activity and lung fluid clearance.Am J Respir Cell Mol Biol. 2013 Aug;49(2):251-9. doi: 10.1165/rcmb.2012-0234OC. Am J Respir Cell Mol Biol. 2013. PMID: 23526224 Free PMC article.
-
Oxidative stress, autophagy and airway ion transport.Am J Physiol Cell Physiol. 2019 Jan 1;316(1):C16-C32. doi: 10.1152/ajpcell.00341.2018. Epub 2018 Oct 10. Am J Physiol Cell Physiol. 2019. PMID: 30303690 Free PMC article. Review.
-
The necessity of NEDD8/Rub1 for vitality and its association with mitochondria-derived oxidative stress.Redox Biol. 2020 Oct;37:101765. doi: 10.1016/j.redox.2020.101765. Epub 2020 Oct 20. Redox Biol. 2020. PMID: 33099217 Free PMC article. Review.
-
Oxygen in the regulation of intestinal epithelial transport.J Physiol. 2014 Jun 15;592(12):2473-89. doi: 10.1113/jphysiol.2013.270249. Epub 2014 Apr 7. J Physiol. 2014. PMID: 24710059 Free PMC article. Review.
-
Oxidized glutathione (GSSG) inhibits epithelial sodium channel activity in primary alveolar epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2015 May 1;308(9):L943-52. doi: 10.1152/ajplung.00213.2014. Epub 2015 Feb 20. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25713321 Free PMC article.
References
-
- Kelly O., Lin C., Ramkumar M., Saxena N. C., Kleyman T. R., Eaton D. C. (2003) Characterization of an amiloride binding region in the α-subunit of ENaC. Am. J. Physiol. Renal Physiol. 285, F1279–F1290 - PubMed
-
- Hummler E., Rossier B. C. (1996) Physiological and pathophysiological role of the epithelial sodium channel in the control of blood pressure. Kidney Blood Press. Res. 19, 160–165 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

