Mechanical stretch inhibits lipopolysaccharide-induced keratinocyte-derived chemokine and tissue factor expression while increasing procoagulant activity in murine lung epithelial cells
- PMID: 23362270
- PMCID: PMC3597825
- DOI: 10.1074/jbc.M112.403220
Mechanical stretch inhibits lipopolysaccharide-induced keratinocyte-derived chemokine and tissue factor expression while increasing procoagulant activity in murine lung epithelial cells
Abstract
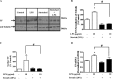
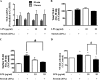
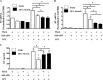
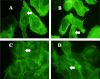
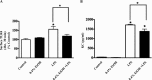
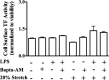
Previous studies have shown that the innate immune stimulant LPS augments mechanical ventilation-induced pulmonary coagulation and inflammation. Whether these effects are mediated by alveolar epithelial cells is unclear. The alveolar epithelium is a key regulator of the innate immune reaction to pathogens and can modulate both intra-alveolar inflammation and coagulation through up-regulation of proinflammatory cytokines and tissue factor (TF), the principal initiator of the extrinsic coagulation pathway. We hypothesized that cyclic mechanical stretch (MS) potentiates LPS-mediated alveolar epithelial cell (MLE-12) expression of the chemokine keratinocyte-derived cytokine (KC) and TF. Contrary to our hypothesis, MS significantly decreased LPS-induced KC and TF mRNA and protein expression. Investigation into potential mechanisms showed that stretch significantly reduced LPS-induced surface expression of TLR4 that was not a result of increased degradation. Decreased cell surface TLR4 expression was concomitant with reduced LPS-mediated NF-κB activation. Immunofluorescence staining showed that cyclic MS markedly altered LPS-induced organization of actin filaments. In contrast to expression, MS significantly increased LPS-induced cell surface TF activity independent of calcium signaling. These findings suggest that cyclic MS of lung epithelial cells down-regulates LPS-mediated inflammatory and procoagulant expression by modulating actin organization and reducing cell surface TLR4 expression and signaling. However, because LPS-induced surface TF activity was enhanced by stretch, these data demonstrate differential pathways regulating TF expression and activity. Ultimately, loss of LPS responsiveness in the epithelium induced by MS could result in increased susceptibility of the lung to bacterial infections in the setting of mechanical ventilation.
Figures


Similar articles
-
NF-κB p65 Knock-down inhibits TF, PAI-1 and promotes activated protein C production in lipopolysaccharide-stimulated alveolar epithelial cells type II.Exp Lung Res. 2018 May-Jun;44(4-5):241-251. doi: 10.1080/01902148.2018.1505975. Epub 2018 Nov 19. Exp Lung Res. 2018. PMID: 30449218
-
Hyaluronan ameliorates LPS-induced acute lung injury in mice via Toll-like receptor (TLR) 4-dependent signaling pathways.Int Immunopharmacol. 2015 Oct;28(2):1050-8. doi: 10.1016/j.intimp.2015.08.021. Epub 2015 Aug 28. Int Immunopharmacol. 2015. PMID: 26321117
-
Ulinastatin Protects Against LPS-Induced Acute Lung Injury By Attenuating TLR4/NF-κB Pathway Activation and Reducing Inflammatory Mediators.Shock. 2018 Nov;50(5):595-605. doi: 10.1097/SHK.0000000000001104. Shock. 2018. PMID: 29324628
-
Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells.J Biol Chem. 2003 Aug 29;278(35):32552-60. doi: 10.1074/jbc.M305536200. Epub 2003 Jun 13. J Biol Chem. 2003. PMID: 12807870
-
Procoagulant activity during viral infections.Front Biosci (Landmark Ed). 2018 Jan 1;23(6):1060-1081. doi: 10.2741/4633. Front Biosci (Landmark Ed). 2018. PMID: 28930589 Review.
Cited by
-
Amphiregulin orchestrates the paracrine immune-suppressive function of amniotic-derived cells through its interplay with COX-2/PGE2/EP4 axis.iScience. 2024 Jul 14;27(8):110508. doi: 10.1016/j.isci.2024.110508. eCollection 2024 Aug 16. iScience. 2024. PMID: 39156643 Free PMC article.
-
Protein kinase R-like endoplasmatic reticulum kinase is a mediator of stretch in ventilator-induced lung injury.Respir Res. 2018 Aug 22;19(1):157. doi: 10.1186/s12931-018-0856-2. Respir Res. 2018. PMID: 30134920 Free PMC article.
-
IL-13 Augments Compressive Stress-Induced Tissue Factor Expression in Human Airway Epithelial Cells.Am J Respir Cell Mol Biol. 2016 Apr;54(4):524-31. doi: 10.1165/rcmb.2015-0252OC. Am J Respir Cell Mol Biol. 2016. PMID: 26407210 Free PMC article.
-
MiR-21-5p modulates LPS-induced acute injury in alveolar epithelial cells by targeting SLC16A10.Sci Rep. 2024 May 15;14(1):11160. doi: 10.1038/s41598-024-61777-x. Sci Rep. 2024. PMID: 38750066 Free PMC article.
-
Nuclear factor-kappa B influences early phase of compensatory lung growth after pneumonectomy in mice.J Biomed Sci. 2017 Jul 5;24(1):41. doi: 10.1186/s12929-017-0350-z. J Biomed Sci. 2017. PMID: 28679393 Free PMC article.
References
-
- Ware L. B., Bastarache J. A., Wang L. (2005) Coagulation and fibrinolysis in human acute lung injury–new therapeutic targets? Keio J. Med. 54, 142–149 - PubMed
-
- Bhadade R. R., de Souza R. A., Harde M. J., Khot A. (2011) Clinical characteristics and outcomes of patients with acute lung injury and ARDS. J. Postgrad. Med. 57, 286–290 - PubMed
-
- Altemeier W. A., Matute-Bello G., Frevert C. W., Kawata Y., Kajikawa O., Martin T. R., Glenny R. W. (2004) Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L533–L542 - PubMed
-
- Altemeier W. A., Matute-Bello G., Gharib S. A., Glenny R. W., Martin T. R., Liles W. C. (2005) Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J. Immunol. 175, 3369–3376 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

