γH2A-binding protein Brc1 affects centromere function in fission yeast
- PMID: 23358415
- PMCID: PMC3624265
- DOI: 10.1128/MCB.01654-12
γH2A-binding protein Brc1 affects centromere function in fission yeast
Abstract
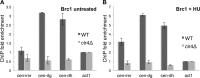
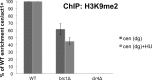
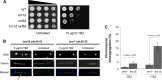
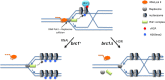
The coordinated replication and transcription of pericentromeric repeats enable RNA interference (RNAi)-mediated transmission of pericentromeric heterochromatin in fission yeast, which is essential for the proper function of centromeres. Rad3/ATR kinase phosphorylates histone H2A on serine-128/-129 to create γH2A in pericentromeric heterochromatin during S phase, which recruits Brc1 through its breast cancer gene 1 protein (BRCA1) C-terminal (BRCT) domains. Brc1 prevents the collapse of stalled replication forks; however, it is unknown whether this activity influences centromere function. Here, we show that Brc1 localizes in pericentromeric heterochromatin during S phase, where it enhances Clr4/Suv39-mediated H3 lysine-9 dimethylation (H3K9me2) and gene silencing. Loss of Brc1 increases sensitivity to the microtubule-destabilizing drug thiabendazole (TBZ) and increases chromosome missegregation in the presence of TBZ. Brc1 retains significant function even when it cannot bind γH2A. However, elimination of the serine-121 site on histone H2A, a target of Bub1 spindle assembly checkpoint kinase, sensitizes γH2A-deficient and brc1Δ cells to replication stress and microtubule destabilization. Collective results suggest that Brc1-mediated stabilization of stalled replication forks is necessary for fully efficient transmission of pericentromeric heterochromatin, which is required for accurate chromosome segregation during mitosis.
Figures


Similar articles
-
Brc1 links replication stress response and centromere function.Cell Cycle. 2013 Jun 1;12(11):1665-71. doi: 10.4161/cc.24900. Epub 2013 May 8. Cell Cycle. 2013. PMID: 23656778 Free PMC article. Review.
-
Continuous requirement for the Clr4 complex but not RNAi for centromeric heterochromatin assembly in fission yeast harboring a disrupted RITS complex.PLoS Genet. 2010 Oct 28;6(10):e1001174. doi: 10.1371/journal.pgen.1001174. PLoS Genet. 2010. PMID: 21060862 Free PMC article.
-
Rad3 decorates critical chromosomal domains with gammaH2A to protect genome integrity during S-Phase in fission yeast.PLoS Genet. 2010 Jul 22;6(7):e1001032. doi: 10.1371/journal.pgen.1001032. PLoS Genet. 2010. PMID: 20661445 Free PMC article.
-
Replication fork stability is essential for the maintenance of centromere integrity in the absence of heterochromatin.Cell Rep. 2013 Mar 28;3(3):638-45. doi: 10.1016/j.celrep.2013.02.007. Epub 2013 Mar 7. Cell Rep. 2013. PMID: 23478021 Free PMC article.
-
Heterochromatin tells CENP-A where to go.Bioessays. 2008 Jun;30(6):526-9. doi: 10.1002/bies.20763. Bioessays. 2008. PMID: 18478529 Review.
Cited by
-
Brc1 Promotes the Focal Accumulation and SUMO Ligase Activity of Smc5-Smc6 during Replication Stress.Mol Cell Biol. 2019 Jan 3;39(2):e00271-18. doi: 10.1128/MCB.00271-18. Print 2019 Jan 15. Mol Cell Biol. 2019. PMID: 30348841 Free PMC article.
-
Mdb1, a fission yeast homolog of human MDC1, modulates DNA damage response and mitotic spindle function.PLoS One. 2014 May 7;9(5):e97028. doi: 10.1371/journal.pone.0097028. eCollection 2014. PLoS One. 2014. PMID: 24806815 Free PMC article.
-
Heterochromatin controls γH2A localization in Neurospora crassa.Eukaryot Cell. 2014 Aug;13(8):990-1000. doi: 10.1128/EC.00117-14. Epub 2014 May 30. Eukaryot Cell. 2014. PMID: 24879124 Free PMC article.
-
Linking replication stress with heterochromatin formation.Chromosoma. 2016 Jun;125(3):523-33. doi: 10.1007/s00412-015-0545-6. Epub 2015 Oct 28. Chromosoma. 2016. PMID: 26511280 Free PMC article. Review.
-
The dark side of centromeres: types, causes and consequences of structural abnormalities implicating centromeric DNA.Nat Commun. 2018 Oct 18;9(1):4340. doi: 10.1038/s41467-018-06545-y. Nat Commun. 2018. PMID: 30337534 Free PMC article. Review.
References
-
- Harper JW, Elledge SJ. 2007. The DNA damage response: ten years after. Mol. Cell 28:739–745 - PubMed
-
- Musacchio A, Salmon ED. 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8:379–393 - PubMed
-
- Cam HP, Sugiyama T, Chen ES, Chen X, FitzGerald PC, Grewal SI. 2005. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat. Genet. 37:809–819 - PubMed
-
- Almeida R, Allshire RC. 2005. RNA silencing and genome regulation. Trends Cell Biol. 15:251–258 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
