Cost-effectiveness of universal serologic screening to prevent nontraumatic hip and vertebral fractures in patients with celiac disease
- PMID: 23357490
- PMCID: PMC3655158
- DOI: 10.1016/j.cgh.2012.12.037
Cost-effectiveness of universal serologic screening to prevent nontraumatic hip and vertebral fractures in patients with celiac disease
Abstract
Background & aims: Patients with asymptomatic or poorly managed celiac disease can experience bone loss, placing them at risk for hip and vertebral fractures. We analyzed the cost-effectiveness of universal serologic screening (USS) vs symptomatic at-risk screening (SAS) strategies for celiac disease because of the risk of nontraumatic hip and vertebral fractures if untreated or undiagnosed.
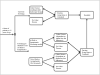
Methods: We developed a lifetime Markov model of the screening strategies, each with male or female cohorts of 1000 patients who were 12 years old when screening began. We screened serum samples for levels of immunoglobulin A, compared with tissue transglutaminase and total immunoglobulin A, and findings were confirmed by mucosal biopsy. Transition probabilities and quality of life estimates were obtained from the literature. We used generalizable cost estimates and Medicare reimbursement rates and ran deterministic and probabilistic sensitivity analyses.
Results: For men, the average lifetime costs were $8532 and $8472 for USS and SAS strategies, respectively, corresponding to average quality-adjusted life year gains of 25.511 and 25.515. Similarly for women, costs were $11,383 and $11,328 for USS and SAS strategies, respectively, corresponding to quality-adjusted life year gains of 25.74 and 25.75. Compared with the current standard of care (SAS), USS produced higher average lifetime costs and lower quality of life for each sex. Deterministic and probabilistic sensitivity analyses showed that the model was robust to realistic changes in all the variables, making USS cost-ineffective on the basis of these outcomes.
Conclusions: USS and SAS are similar in lifetime costs and quality of life, although the current SAS strategy was overall more cost-effective in preventing bone loss and fractures among patients with undiagnosed or subclinical disease. On the basis of best available supportive evidence, it is more cost-effective to maintain the standard celiac screening practices, although future robust population-based evidence in other health outcomes could be leveraged to reevaluate current screening guidelines.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Genetic testing before serologic screening in relatives of patients with celiac disease as a cost containment method.J Clin Gastroenterol. 2009 Jan;43(1):43-50. doi: 10.1097/MCG.0b013e318187311d. J Clin Gastroenterol. 2009. PMID: 19020464
-
Serologic testing for celiac disease in young adults--a cost-effect analysis.Dig Dis Sci. 2005 Apr;50(4):796-805. doi: 10.1007/s10620-005-2576-y. Dig Dis Sci. 2005. PMID: 15844721
-
Screening for celiac disease in asymptomatic children with Down syndrome: cost-effectiveness of preventing lymphoma.Pediatrics. 2006 Aug;118(2):594-602. doi: 10.1542/peds.2005-2123. Pediatrics. 2006. PMID: 16882812
-
Screening for Celiac Disease: Evidence Report and Systematic Review for the US Preventive Services Task Force.JAMA. 2017 Mar 28;317(12):1258-1268. doi: 10.1001/jama.2016.10395. JAMA. 2017. PMID: 28350935 Review.
-
Should adults be screened for celiac disease? What are the benefits and harms of screening?Gastroenterology. 2005 Apr;128(4 Suppl 1):S104-8. doi: 10.1053/j.gastro.2005.02.021. Gastroenterology. 2005. PMID: 15825117 Review.
Cited by
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Is mass screening for coeliac disease a wise use of resources? A health economic evaluation.BMC Gastroenterol. 2021 Apr 9;21(1):159. doi: 10.1186/s12876-021-01737-1. BMC Gastroenterol. 2021. PMID: 33836647 Free PMC article.
-
Systematic Literature Review of the Economic Burden of Celiac Disease.Pharmacoeconomics. 2019 Jan;37(1):45-61. doi: 10.1007/s40273-018-0707-5. Pharmacoeconomics. 2019. PMID: 30221333
-
The impact of diagnosis on health-related quality of life in people with coeliac disease: a UK population-based longitudinal perspective.BMC Gastroenterol. 2019 May 2;19(1):68. doi: 10.1186/s12876-019-0980-6. BMC Gastroenterol. 2019. PMID: 31046685 Free PMC article.
-
Screening for celiac disease in the general population and in high-risk groups.United European Gastroenterol J. 2015 Apr;3(2):106-20. doi: 10.1177/2050640614561668. United European Gastroenterol J. 2015. PMID: 25922671 Free PMC article. Review.
References
-
- Haas SV. Celiac disease, its specific treatment and cure without nutritional relapse. JAMA. 1932;99:448.
-
- Booth CC. History of celiac disease. BMJ. 1989:298–527. - PubMed
-
- Bottaro G, Cataldo F, Rotolo N, Spina M, Corazza GR. The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol. 1999 Mar;94(3):691–6. - PubMed
-
- Holmes GK. Non-malignant complications of coeliac disease. Acta Paediatr Suppl. 1996 May;412:68–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

