Magnetic shielding accelerates the proliferation of human neuroblastoma cell by promoting G1-phase progression
- PMID: 23355897
- PMCID: PMC3552807
- DOI: 10.1371/journal.pone.0054775
Magnetic shielding accelerates the proliferation of human neuroblastoma cell by promoting G1-phase progression
Abstract
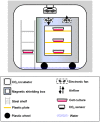
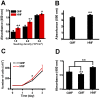
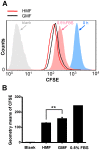
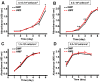
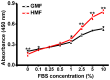
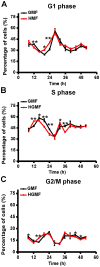
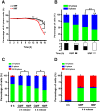
Organisms have been exposed to the geomagnetic field (GMF) throughout evolutionary history. Exposure to the hypomagnetic field (HMF) by deep magnetic shielding has recently been suggested to have a negative effect on the structure and function of the central nervous system, particularly during early development. Although changes in cell growth and differentiation have been observed in the HMF, the effects of the HMF on cell cycle progression still remain unclear. Here we show that continuous HMF exposure significantly increases the proliferation of human neuroblastoma (SH-SY5Y) cells. The acceleration of proliferation results from a forward shift of the cell cycle in G1-phase. The G2/M-phase progression is not affected in the HMF. Our data is the first to demonstrate that the HMF can stimulate the proliferation of SH-SY5Y cells by promoting cell cycle progression in the G1-phase. This provides a novel way to study the mechanism of cells in response to changes of environmental magnetic field including the GMF.
Conflict of interest statement
Figures

Similar articles
-
Shielding of the geomagnetic field reduces hydrogen peroxide production in human neuroblastoma cell and inhibits the activity of CuZn superoxide dismutase.Protein Cell. 2017 Jul;8(7):527-537. doi: 10.1007/s13238-017-0403-9. Epub 2017 Apr 26. Protein Cell. 2017. PMID: 28447293 Free PMC article.
-
Shielded geomagnetic field accelerates glucose consumption in human neuroblastoma cells by promoting anaerobic glycolysis.Biochem Biophys Res Commun. 2022 Apr 23;601:101-108. doi: 10.1016/j.bbrc.2022.01.114. Epub 2022 Jan 31. Biochem Biophys Res Commun. 2022. PMID: 35240496
-
Shielding of the Geomagnetic Field Alters Actin Assembly and Inhibits Cell Motility in Human Neuroblastoma Cells.Sci Rep. 2016 Mar 31;6:22624. doi: 10.1038/srep22624. Sci Rep. 2016. PMID: 27029216 Free PMC article.
-
Biological Effects of Hypomagnetic Field: Ground-Based Data for Space Exploration.Bioelectromagnetics. 2021 Sep;42(6):516-531. doi: 10.1002/bem.22360. Epub 2021 Jul 10. Bioelectromagnetics. 2021. PMID: 34245597 Review.
-
Biological Effects of Space Hypomagnetic Environment on Circadian Rhythm.Front Physiol. 2021 Mar 9;12:643943. doi: 10.3389/fphys.2021.643943. eCollection 2021. Front Physiol. 2021. PMID: 33767637 Free PMC article. Review.
Cited by
-
Human Ischaemic Cascade Studies Using SH-SY5Y Cells: a Systematic Review and Meta-Analysis.Transl Stroke Res. 2018 Dec;9(6):564-574. doi: 10.1007/s12975-018-0620-4. Epub 2018 Mar 23. Transl Stroke Res. 2018. PMID: 29572690 Free PMC article. Review.
-
Regulation of Osteoblast Differentiation and Iron Content in MC3T3-E1 Cells by Static Magnetic Field with Different Intensities.Biol Trace Elem Res. 2018 Jul;184(1):214-225. doi: 10.1007/s12011-017-1161-5. Epub 2017 Oct 19. Biol Trace Elem Res. 2018. PMID: 29052173 Free PMC article.
-
Hypomagnetic Conditions and Their Biological Action (Review).Biology (Basel). 2023 Dec 11;12(12):1513. doi: 10.3390/biology12121513. Biology (Basel). 2023. PMID: 38132339 Free PMC article. Review.
-
Magnetic Force-Based Microfluidic Techniques for Cellular and Tissue Bioengineering.Front Bioeng Biotechnol. 2018 Dec 19;6:192. doi: 10.3389/fbioe.2018.00192. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 30619842 Free PMC article. Review.
-
Shielding of the geomagnetic field reduces hydrogen peroxide production in human neuroblastoma cell and inhibits the activity of CuZn superoxide dismutase.Protein Cell. 2017 Jul;8(7):527-537. doi: 10.1007/s13238-017-0403-9. Epub 2017 Apr 26. Protein Cell. 2017. PMID: 28447293 Free PMC article.
References
-
- Lohmann KJ (2010) Q&A: Animal behaviour: Magnetic-field perception. Nature 464: 1140–1142. - PubMed
-
- Jogler C, Schüler D (2009) Genomics, genetics, and cell biology of magnetosome formation. Annu Rev Microbiol 63: 501–21. - PubMed
-
- Dubrov AP (1989) The geomagnetic field and life: Geomagnetobiology. New York: Plenum. (Translated from Russian by Sinclair FL).
-
- Belyavskaya NA (2004) Biological effects due to weak magnetic field on plants. Adv Space Res 34: 1566–1574. - PubMed
-
- Jia B, Shang P (2009) Research progress of biological effects of hypomagnetic fields. Space Med Med Eng 22: 308–312.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

