Revealing the secrets of neuronal circuits with recombinant rabies virus technology
- PMID: 23355811
- PMCID: PMC3553424
- DOI: 10.3389/fncir.2013.00002
Revealing the secrets of neuronal circuits with recombinant rabies virus technology
Abstract
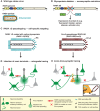
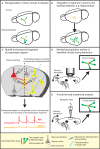
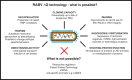
An understanding of how the brain processes information requires knowledge of the architecture of its underlying neuronal circuits, as well as insights into the relationship between architecture and physiological function. A range of sophisticated tools is needed to acquire this knowledge, and recombinant rabies virus (RABV) is becoming an increasingly important part of this essential toolbox. RABV has been recognized for years for its properties as a synapse-specific trans-neuronal tracer. A novel genetically modified variant now enables the investigation of specific monosynaptic connections. This technology, in combination with other genetic, physiological, optical, and computational tools, has enormous potential for the visualization of neuronal circuits, and for monitoring and manipulating their activity. Here we will summarize the latest developments in this fast moving field and provide a perspective for the use of this technology for the dissection of neuronal circuit structure and function in the normal and diseased brain.
Keywords: connectivity; monosynaptic; neuronal tracer; synapses; wiring diagram.
Figures

Similar articles
-
Multiplex Neural Circuit Tracing With G-Deleted Rabies Viral Vectors.Front Neural Circuits. 2020 Jan 10;13:77. doi: 10.3389/fncir.2019.00077. eCollection 2019. Front Neural Circuits. 2020. PMID: 31998081 Free PMC article.
-
Recombinant Fluorescent Rabies Virus Vectors for Tracing Neurons and Synaptic Connections.Cold Spring Harb Protoc. 2015 Dec 2;2015(12):pdb.top089391. doi: 10.1101/pdb.top089391. Cold Spring Harb Protoc. 2015. PMID: 26631133
-
Dual Anterograde and Retrograde Viral Tracing of Reciprocal Connectivity.Methods Mol Biol. 2017;1538:321-340. doi: 10.1007/978-1-4939-6688-2_21. Methods Mol Biol. 2017. PMID: 27943199
-
Trans-synaptic Neural Circuit-Tracing with Neurotropic Viruses.Neurosci Bull. 2019 Oct;35(5):909-920. doi: 10.1007/s12264-019-00374-9. Epub 2019 Apr 19. Neurosci Bull. 2019. PMID: 31004271 Free PMC article. Review.
-
Rabies virus-mediated connectivity tracing from single neurons.J Neurosci Methods. 2019 Sep 1;325:108365. doi: 10.1016/j.jneumeth.2019.108365. Epub 2019 Jul 19. J Neurosci Methods. 2019. PMID: 31330160 Review.
Cited by
-
Effects of G-gene Deletion and Replacement on Rabies Virus Vector Gene Expression.PLoS One. 2015 May 29;10(5):e0128020. doi: 10.1371/journal.pone.0128020. eCollection 2015. PLoS One. 2015. PMID: 26023771 Free PMC article.
-
Current Genetic Techniques in Neural Circuit Control of Feeding and Energy Metabolism.Adv Exp Med Biol. 2018;1090:211-233. doi: 10.1007/978-981-13-1286-1_12. Adv Exp Med Biol. 2018. PMID: 30390293 Free PMC article. Review.
-
Patch-Seq Protocol to Analyze the Electrophysiology, Morphology and Transcriptome of Whole Single Neurons Derived From Human Pluripotent Stem Cells.Front Mol Neurosci. 2018 Aug 10;11:261. doi: 10.3389/fnmol.2018.00261. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30147644 Free PMC article.
-
Synaptic circuit organization of motor corticothalamic neurons.J Neurosci. 2015 Feb 4;35(5):2293-307. doi: 10.1523/JNEUROSCI.4023-14.2015. J Neurosci. 2015. PMID: 25653383 Free PMC article.
-
Neuronal replacement: Concepts, achievements, and call for caution.Curr Opin Neurobiol. 2021 Aug;69:185-192. doi: 10.1016/j.conb.2021.03.014. Epub 2021 May 10. Curr Opin Neurobiol. 2021. PMID: 33984604 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

