CYP3A5 gene variation influences cyclosporine A metabolite formation and renal cyclosporine disposition
- PMID: 23354298
- PMCID: PMC3604156
- DOI: 10.1097/TP.0b013e31827e6ad9
CYP3A5 gene variation influences cyclosporine A metabolite formation and renal cyclosporine disposition
Abstract
Background: Higher concentrations of AM19 and AM1c9, secondary metabolites of cyclosporine A (CsA), have been associated with nephrotoxicity in organ transplant patients. The risk of renal toxicity may depend on the accumulation of CsA and its metabolites in the renal tissue. We evaluated the hypothesis that CYP3A5 genotype, and inferred enzyme expression, affects systemic CsA metabolite exposure and intrarenal CsA accumulation.
Methods: An oral dose of CsA was administered to 24 healthy volunteers who were selected based on their CYP3A5 genotype. CsA and its six main metabolites in whole blood and urine were measured by liquid chromatography-mass spectometry. In vitro incubations of CsA, AM1, AM9, and AM1c with recombinant CYP3A4 and CYP3A5 were performed to evaluate the formation pathways of AM19 and AM1c9.
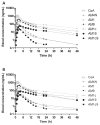
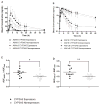
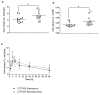
Results: The mean CsA oral clearance was similar between CYP3A5 expressors and nonexpressors. However, compared with CYP3A5 nonexpressors, the average blood area under the concentration-time curve (AUC) for AM19 and AM1c9 was 47.4% and 51.3% higher in CYP3A5 expressors (P=0.040 and 0.011, respectively), corresponding to 30% higher AUCmetabolite/AUCCsA ratios for AM19 and AM1c9 in CYP3A5 expressors. The mean apparent urinary CsA clearance based on a 48-hr collection was 20.4% lower in CYP3A5 expressors compared with CYP3A5 nonexpressors (4.2±1.0 and 5.3±1.3 mL/min, respectively; P=0.037), which is suggestive of CYP3A5-dependent intrarenal CsA metabolism.
Conclusions: At steady state, intrarenal accumulation of CsA and its secondary metabolites should depend on the CYP3A5 genotype of the liver and kidneys. This may contribute to interpatient variability in the risk of CsA-induced nephrotoxicity.
Conflict of interest statement
No conflict of interest
Figures
Similar articles
-
In vitro metabolism of cyclosporine A by human kidney CYP3A5.Biochem Pharmacol. 2004 Nov 1;68(9):1889-902. doi: 10.1016/j.bcp.2004.07.012. Biochem Pharmacol. 2004. PMID: 15450954
-
Measurement and compartmental modeling of the effect of CYP3A5 gene variation on systemic and intrarenal tacrolimus disposition.Clin Pharmacol Ther. 2012 Dec;92(6):737-45. doi: 10.1038/clpt.2012.175. Epub 2012 Oct 17. Clin Pharmacol Ther. 2012. PMID: 23073208 Free PMC article.
-
The concentration of cyclosporine metabolites is significantly lower in kidney transplant recipients with diabetes mellitus.Ther Drug Monit. 2012 Feb;34(1):38-45. doi: 10.1097/FTD.0b013e318241ac71. Ther Drug Monit. 2012. PMID: 22210099
-
A systematic review of the effect of CYP3A5 genotype on the apparent oral clearance of tacrolimus in renal transplant recipients.Ther Drug Monit. 2010 Dec;32(6):708-14. doi: 10.1097/FTD.0b013e3181f3c063. Ther Drug Monit. 2010. PMID: 20864901 Review.
-
Metabolic Pathway of Cyclosporine A and Its Correlation with Nephrotoxicity.Curr Drug Metab. 2019;20(2):84-90. doi: 10.2174/1389200219666181031113505. Curr Drug Metab. 2019. PMID: 30378493 Review.
Cited by
-
Pharmacogenetics and drug-induced nephrotoxicity in renal transplant recipients.Bioimpacts. 2015;5(1):45-54. doi: 10.15171/bi.2015.12. Epub 2015 Feb 21. Bioimpacts. 2015. PMID: 25901296 Free PMC article. Review.
-
The influence of CYP3A, PPARA, and POR genetic variants on the pharmacokinetics of tacrolimus and cyclosporine in renal transplant recipients.Eur J Clin Pharmacol. 2014 Jun;70(6):685-93. doi: 10.1007/s00228-014-1656-3. Eur J Clin Pharmacol. 2014. PMID: 24658827 Free PMC article.
-
Clinical implementation of pharmacogenetics in kidney transplantation: calcineurin inhibitors in the starting blocks.Br J Clin Pharmacol. 2014 Apr;77(4):715-28. doi: 10.1111/bcp.12253. Br J Clin Pharmacol. 2014. PMID: 24118098 Free PMC article. Review.
-
Antisense oligonucleotide development for the selective modulation of CYP3A5 in renal disease.Sci Rep. 2021 Feb 25;11(1):4722. doi: 10.1038/s41598-021-84194-w. Sci Rep. 2021. PMID: 33633318 Free PMC article.
-
Role of pharmacogenomics in dialysis and transplantation.Curr Opin Nephrol Hypertens. 2014 Nov;23(6):570-7. doi: 10.1097/MNH.0000000000000065. Curr Opin Nephrol Hypertens. 2014. PMID: 25162201 Free PMC article.
References
-
- Calne RY, White DJ, Thiru S, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;2 (8104–5):1323. - PubMed
-
- Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311 (11):699. - PubMed
-
- Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349 (24):2326. - PubMed
-
- Naesens M, Kuypers D, Sarwal MM. The bumpy road of genomic medicine in transplantation: lessons from studies on calcineurin inhibitor nephrotoxicity. Transplantation. 2012;93 (6):578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

