Dichotomy of decorin activity on the insulin-like growth factor-I system
- PMID: 23351020
- PMCID: PMC3651761
- DOI: 10.1111/febs.12149
Dichotomy of decorin activity on the insulin-like growth factor-I system
Abstract
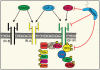
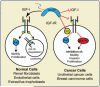
The stromal-specific proteoglycan decorin has emerged in recent years as a critical regulator of tumor initiation and progression. Decorin regulates the biology of various types of cancer by modulating the activity of several receptor tyrosine kinases coordinating growth, survival, migration, and angiogenesis. Decorin binds to surface receptors for epidermal growth factor and hepatocyte growth factor with high affinity, and negatively regulates their activity and signaling via robust internalization and eventual degradation. The insulin-like growth factor (IGF)-I system plays a critical role in the regulation of cell growth both in vivo and in vitro. The IGF-I receptor (IGF-IR) is also essential for cellular transformation, owing to its ability to enhance cell proliferation and protect cancer cells from apoptosis. Recent data have pointed to a role of decorin in regulating the IGF-I system in both nontransformed and transformed cells. Significantly, there is a surprising dichotomy in the mechanism of decorin action on IGF-IR signaling, which differs considerably between physiological and pathological cellular models. In this review, we summarize the current knowledge on decorin regulation of the IGF-I system in normal and transformed cells, and discuss possible decorin-based therapeutic approaches to target IGF-IR-driven tumors.
© 2013 The Authors Journal compilation © 2013 FEBS.
Figures

Similar articles
-
Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling.J Biol Chem. 2011 Oct 7;286(40):34712-21. doi: 10.1074/jbc.M111.262766. Epub 2011 Aug 12. J Biol Chem. 2011. PMID: 21840990 Free PMC article.
-
Decorin differentially modulates the activity of insulin receptor isoform A ligands.Matrix Biol. 2014 Apr;35:82-90. doi: 10.1016/j.matbio.2013.12.010. Epub 2014 Jan 2. Matrix Biol. 2014. PMID: 24389353 Free PMC article.
-
Decorin inhibits the insulin-like growth factor I signaling in bone marrow mesenchymal stem cells of aged humans.Aging (Albany NY). 2020 Nov 26;13(1):578-597. doi: 10.18632/aging.202166. Epub 2020 Nov 26. Aging (Albany NY). 2020. PMID: 33257596 Free PMC article.
-
The IGF axis and hepatocarcinogenesis.Mol Pathol. 2001 Jun;54(3):138-44. doi: 10.1136/mp.54.3.138. Mol Pathol. 2001. PMID: 11376124 Free PMC article. Review.
-
Insulin-like growth factors in endometrial function.Gynecol Endocrinol. 1998 Dec;12(6):399-406. doi: 10.3109/09513599809012842. Gynecol Endocrinol. 1998. PMID: 10065165 Review.
Cited by
-
The Insulin-like Growth Factor System and Colorectal Cancer.Life (Basel). 2022 Aug 20;12(8):1274. doi: 10.3390/life12081274. Life (Basel). 2022. PMID: 36013453 Free PMC article. Review.
-
Decorin induces mitophagy in breast carcinoma cells via peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and mitostatin.J Biol Chem. 2014 Feb 21;289(8):4952-68. doi: 10.1074/jbc.M113.512566. Epub 2014 Jan 8. J Biol Chem. 2014. PMID: 24403067 Free PMC article.
-
Overexpression of decorin promoted angiogenesis in diabetic cardiomyopathy via IGF1R-AKT-VEGF signaling.Sci Rep. 2017 Mar 14;7:44473. doi: 10.1038/srep44473. Sci Rep. 2017. PMID: 28290552 Free PMC article.
-
Discoidin Domain Receptor 1 functionally interacts with the IGF-I system in bladder cancer.Matrix Biol Plus. 2020 Jan 17;6-7:100022. doi: 10.1016/j.mbplus.2020.100022. eCollection 2020 May. Matrix Biol Plus. 2020. PMID: 33543020 Free PMC article.
-
Decorin causes autophagy in endothelial cells via Peg3.Proc Natl Acad Sci U S A. 2013 Jul 9;110(28):E2582-91. doi: 10.1073/pnas.1305732110. Epub 2013 Jun 24. Proc Natl Acad Sci U S A. 2013. PMID: 23798385 Free PMC article.
References
-
- Iozzo RV, Goldoni S, Berendsen A, Young MF. Small leucine-rich proteoglycans. In: Mecham RP, editor. Extracellular Matrix: An overview. Springer; 2011. pp. 197–231.
-
- Merline R, Nastase MV, Iozzo RV, Schaefer L. Small Leucine-rich proteoglycans: multifunctional signaling effectors. In: Karamanos N, editor. Extracellular Matrix: Pathobiology and signaling. Walter de Gruytier Gmbh and Co.; Berlin: 2012. pp. 185–196.
-
- Danielson KG, Fazzio A, Cohen I, Cannizzaro LA, Eichstetter I, Iozzo RV. The human decorin gene: intron-exon organization, discovery of two alternatively spliced exons in the 5′ untranslated region, and mapping of the gene to chromosome 12q23. Genomics. 1993;15:146–160. - PubMed
-
- Santra M, Danielson KG, Iozzo RV. Structural and functional characterization of the human decorin gene promoter. A homopurine-homopyrimidine S1 nuclease-sensitive region is involved in transcriptional control. J Biol Chem. 1994;269:579–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

