Characterization of adult α- and β-globin elevated by hydrogen peroxide in cervical cancer cells that play a cytoprotective role against oxidative insults
- PMID: 23349856
- PMCID: PMC3547883
- DOI: 10.1371/journal.pone.0054342
Characterization of adult α- and β-globin elevated by hydrogen peroxide in cervical cancer cells that play a cytoprotective role against oxidative insults
Abstract
Objectives: Hemoglobin (Hgb) is the main oxygen and carbon dioxide carrier in cells of erythroid lineage and is responsible for oxygen delivery to the respiring tissues of the body. However, Hgb is also expressed in nonerythroid cells. In the present study, the expression of Hgb in human uterine cervix carcinoma cells and its role in cervical cancer were investigated.
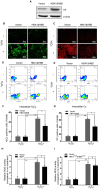
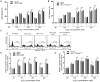
Methodology: The expression level of Hgb in cervical cancer tissues was assessed by quantitative reverse transcriptase-PCR (qRT-PCR). We applied multiple methods, such as RT-PCR, immunoblotting, and immunohistochemical analysis, to confirm Hgb expression in cervical cancer cells. The effects of ectopic expression of Hgb and Hgb mutants on oxidative stress and cell viability were investigated by cellular reactive oxygen species (ROS) analysis and lactate dehydrogenase (LDH) array, respectively. Both Annexin V staining assay by flow cytometry and caspase-3 activity assay were used, respectively, to evaluate cell apoptosis.
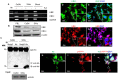
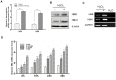
Results: qRT-PCR analysis showed that Hgb-α- (HBA1) and Hgb-β-globin (HBB) gene expression was significantly higher in cervical carcinoma than in normal cervical tissues, whereas the expression of hematopoietic transcription factors and erythrocyte specific marker genes was not increased. Immunostaining experiments confirmed the expression of Hgb in cancer cells of the uterine cervix. Hgb mRNA and protein were also detected in the human cervical carcinoma cell lines SiHa and CaSki, and Hgb expression was up-regulated by hydrogen peroxide-induced oxidative stress. Importantly, ectopic expression of wild type HBA1/HBB or HBA1, rather than mutants HBA1(H88R)/HBB(H93R) unable to bind hemo, suppressed oxidative stress and improved cell viability.
Conclusions: The present findings show for the first time that Hgb is expressed in cervical carcinoma cells and may act as an antioxidant, attenuating oxidative stress-induced damage in cervical cancer cells. These data provide a significant impact not only in globin biology but also in understanding of cervical cancer pathogenesis associated with oxidative stress.
Conflict of interest statement
Figures


Similar articles
-
Upregulation of hemoglobin expression by oxidative stress in hepatocytes and its implication in nonalcoholic steatohepatitis.PLoS One. 2011;6(9):e24363. doi: 10.1371/journal.pone.0024363. Epub 2011 Sep 12. PLoS One. 2011. PMID: 21931690 Free PMC article.
-
Role of p53 in antioxidant defense of HPV-positive cervical carcinoma cells following H2O2 exposure.J Cell Sci. 2007 Jul 1;120(Pt 13):2284-94. doi: 10.1242/jcs.002345. Epub 2007 Jun 13. J Cell Sci. 2007. PMID: 17567683
-
CYT-Rx20 Inhibits Cervical Cancer Cell Growth and Migration Through Oxidative Stress-Induced DNA Damage, Cell Apoptosis, and Epithelial-to-Mesenchymal Transition Inhibition.Int J Gynecol Cancer. 2017 Sep;27(7):1306-1317. doi: 10.1097/IGC.0000000000001033. Int J Gynecol Cancer. 2017. PMID: 30814237
-
Ten Years of Routine α- and β-Globin Gene Sequencing in UK Hemoglobinopathy Referrals Reveals 60 Novel Mutations.Hemoglobin. 2016;40(2):75-84. doi: 10.3109/03630269.2015.1113990. Epub 2015 Dec 4. Hemoglobin. 2016. PMID: 26635043 Review.
-
Oxidative stress: therapeutic approaches for cervical cancer treatment.Clinics (Sao Paulo). 2018 Dec 10;73(suppl 1):e548s. doi: 10.6061/clinics/2018/e548s. Clinics (Sao Paulo). 2018. PMID: 30540121 Free PMC article. Review.
Cited by
-
Exploring unconventional attributes of red blood cells and their potential applications in biomedicine.Protein Cell. 2024 May 7;15(5):315-330. doi: 10.1093/procel/pwae001. Protein Cell. 2024. PMID: 38270470 Free PMC article. No abstract available.
-
Hemoglobin alpha is a redox-sensitive mitochondrial-related protein in T-lymphocytes.bioRxiv [Preprint]. 2024 Sep 20:2024.09.16.613298. doi: 10.1101/2024.09.16.613298. bioRxiv. 2024. Update in: Free Radic Biol Med. 2025 Feb 1;227:1-11. doi: 10.1016/j.freeradbiomed.2024.11.044. PMID: 39345360 Free PMC article. Updated. Preprint.
-
Molecular Genetics of Lidocaine-Containing Cardioplegia in the Human Heart During Cardiac Surgery.Ann Thorac Surg. 2018 Nov;106(5):1379-1387. doi: 10.1016/j.athoracsur.2018.06.016. Epub 2018 Jul 17. Ann Thorac Surg. 2018. PMID: 30028983 Free PMC article.
-
Effect of the dietary supplementation with extracts of chestnut wood and grape pomace on performance and jejunum response in female and male broiler chickens at different ages.J Anim Sci Biotechnol. 2022 Aug 17;13(1):102. doi: 10.1186/s40104-022-00736-w. J Anim Sci Biotechnol. 2022. PMID: 35978386 Free PMC article.
-
Advanced Proteogenomic Analysis Reveals Multiple Peptide Mutations and Complex Immunoglobulin Peptides in Colon Cancer.J Proteome Res. 2015 Sep 4;14(9):3555-67. doi: 10.1021/acs.jproteome.5b00264. Epub 2015 Jul 21. J Proteome Res. 2015. PMID: 26139413 Free PMC article.
References
-
- Wittenberg BA, Wittenberg JB (1989) Transport of oxygen in muscle. Annu Rev Physiol 51: 857–878. - PubMed
-
- Schmidt M, Gerlach F, Avivi A, Laufs T, Wystub S, et al. (2004) Cytoglobin is a respiratory protein in connective tissue and neurons, which is up-regulated by hypoxia. Journal of Biological Chemistry 279: 8063–8069. - PubMed
-
- Burmester T, Weich B, Reinhardt S, Hankeln T (2000) A vertebrate globin expressed in the brain. Nature 407: 520–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

