Blueprints of signaling interactions between pattern recognition receptors: implications for the design of vaccine adjuvants
- PMID: 23345580
- PMCID: PMC3592350
- DOI: 10.1128/CVI.00703-12
Blueprints of signaling interactions between pattern recognition receptors: implications for the design of vaccine adjuvants
Abstract
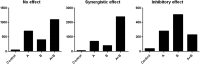
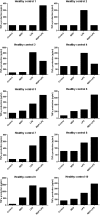
Innate immunity activation largely depends on recognition of microorganism structures by Pattern Recognition Receptors (PRRs). PRR downstream signaling results in production of pro- and anti-inflammatory cytokines and other mediators. Moreover, PRR engagement in antigen-presenting cells initiates the activation of adaptive immunity. Recent reports suggest that for the activation of innate immune responses and initiation of adaptive immunity, synergistic effects between two or more PRRs are necessary. No systematic analysis of the interaction between the major PRR pathways were performed to date. In this study, a systematical analysis of the interactions between PRR signaling pathways was performed. PBMCs derived from 10 healthy volunteers were stimulated with either a single PRR ligand or a combination of two PRR ligands. Known ligands for the major PRR families were used: Toll-like receptors (TLRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs), and RigI-helicases. After 24 h of incubation, production of tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), IL-6, and IL-10 was measured in supernatants by enzyme-linked immunosorbent assay (ELISA). The consistency of the PRR interactions (both inhibitory and synergistic) between the various individuals was assessed. A number of PRR-dependent signaling interactions were found to be consistent, both between individuals and with regard to multiple cytokines. The combinations of TLR2 and NOD2, TLR5 and NOD2, TLR5 and TLR3, and TLR5 and TLR9 acted as synergistic combinations. Surprisingly, inhibitory interactions between TLR4 and TLR2, TLR4 and Dectin-1, and TLR2 and TLR9 as well as TLR3 and TLR2 were observed. These consistent signaling interactions between PRR combinations may represent promising targets for immunomodulation and vaccine adjuvant development.
Figures


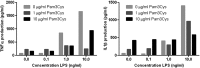
Similar articles
-
Powerful Complex Immunoadjuvant Based on Synergistic Effect of Combined TLR4 and NOD2 Activation Significantly Enhances Magnitude of Humoral and Cellular Adaptive Immune Responses.PLoS One. 2016 May 17;11(5):e0155650. doi: 10.1371/journal.pone.0155650. eCollection 2016. PLoS One. 2016. PMID: 27187797 Free PMC article.
-
Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants.Proc Natl Acad Sci U S A. 2014 Aug 26;111(34):12294-9. doi: 10.1073/pnas.1400478111. Epub 2014 Aug 18. Proc Natl Acad Sci U S A. 2014. PMID: 25136133 Free PMC article. Review.
-
Expression of TRAF6 and pro-inflammatory cytokines through activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal ligament fibroblasts.Arch Oral Biol. 2011 Oct;56(10):1064-72. doi: 10.1016/j.archoralbio.2011.02.020. Epub 2011 Mar 31. Arch Oral Biol. 2011. PMID: 21457942
-
Interaction of Pattern Recognition Receptors with Mycobacterium Tuberculosis.J Clin Immunol. 2015 Jan;35(1):1-10. doi: 10.1007/s10875-014-0103-7. Epub 2014 Oct 14. J Clin Immunol. 2015. PMID: 25312698 Free PMC article. Review.
-
HIV-1-exposed seronegative individuals show alteration in TLR expression and pro-inflammatory cytokine production ex vivo: An innate immune quiescence status?Immunol Res. 2016 Feb;64(1):280-90. doi: 10.1007/s12026-015-8748-8. Immunol Res. 2016. PMID: 26616295
Cited by
-
A unique combination adjuvant modulates immune responses preventing vaccine-enhanced pulmonary histopathology after a single dose vaccination with fusion protein and challenge with respiratory syncytial virus.Virology. 2019 Aug;534:1-13. doi: 10.1016/j.virol.2019.05.010. Epub 2019 May 28. Virology. 2019. PMID: 31163351 Free PMC article.
-
Targeted Programming of the Lymph Node Environment Causes Evolution of Local and Systemic Immunity.Cell Mol Bioeng. 2016;9:418-432. doi: 10.1007/s12195-016-0455-6. Epub 2016 Jun 27. Cell Mol Bioeng. 2016. PMID: 27547269 Free PMC article.
-
Differences in innate cytokine responses between European and African children.PLoS One. 2014 Apr 17;9(4):e95241. doi: 10.1371/journal.pone.0095241. eCollection 2014. PLoS One. 2014. PMID: 24743542 Free PMC article. Clinical Trial.
-
A cell-based microarray to investigate combinatorial effects of microparticle-encapsulated adjuvants on dendritic cell activation.J Mater Chem B. 2016 Mar 7;4(9):1672-1685. doi: 10.1039/C5TB01754H. Epub 2015 Sep 30. J Mater Chem B. 2016. PMID: 26985393 Free PMC article.
-
Review: Current trends, challenges, and success stories in adjuvant research.Front Immunol. 2023 Jan 19;14:1105655. doi: 10.3389/fimmu.2023.1105655. eCollection 2023. Front Immunol. 2023. PMID: 36742311 Free PMC article. Review.
References
-
- Bourhis LL, Werts C. 2007. Role of Nods in bacterial infection. Microbes Infect. 9: 629– 636 - PubMed
-
- Testro AG, Visvanathan K. 2009. Toll-like receptors and their role in gastrointestinal disease. J. Gastroenterol. Hepatol. 24: 943– 954 - PubMed
-
- Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J. 2007. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J. Immunol. 178: 1164– 1171 - PubMed
-
- Fitzgerald KA, Chen ZJ. 2006. Sorting out Toll signals. Cell 125: 834– 836 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

