CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study
- PMID: 23343832
- PMCID: PMC3700465
- DOI: 10.1182/blood-2012-10-461848
CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study
Abstract
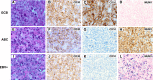
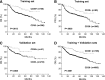
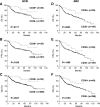
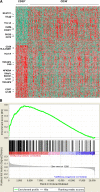
CD30, originally identified as a cell-surface marker of Reed-Sternberg and Hodgkin cells of classical Hodgkin lymphoma, is also expressed by several types of non-Hodgkin lymphoma, including a subset of diffuse large B-cell lymphoma (DLBCL). However, the prognostic and biological importance of CD30 expression in DLBCL is unknown. Here we report that CD30 expression is a favorable prognostic factor in a cohort of 903 de novo DLBCL patients. CD30 was expressed in ∼14% of DLBCL patients. Patients with CD30(+) DLBCL had superior 5-year overall survival (CD30(+), 79% vs CD30(-), 59%; P = .001) and progression-free survival (P = .003). The favorable outcome of CD30 expression was maintained in both the germinal center B-cell and activated B-cell subtypes. Gene expression profiling revealed the upregulation of genes encoding negative regulators of nuclear factor κB activation and lymphocyte survival, and downregulation of genes encoding B-cell receptor signaling and proliferation, as well as prominent cytokine and stromal signatures in CD30(+) DLBCL patients, suggesting a distinct molecular basis for its favorable outcome. Given the superior prognostic value, unique gene expression signature, and significant value of CD30 as a therapeutic target for brentuximab vedotin in ongoing successful clinical trials, it seems appropriate to consider CD30(+) DLBCL as a distinct subgroup of DLBCL.
Figures

Comment in
-
CD30, another useful predictor of survival in DLBCL?Blood. 2013 Apr 4;121(14):2582-3. doi: 10.1182/blood-2013-02-481978. Blood. 2013. PMID: 23557969 No abstract available.
Similar articles
-
Prevalence and clinicopathologic features of CD30-positive de novo diffuse large B-cell lymphoma in Chinese patients: a retrospective study of 232 cases.Int J Clin Exp Pathol. 2015 Dec 1;8(12):15825-35. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26884853 Free PMC article.
-
CD30 expression in de novo diffuse large B-cell lymphoma: a population-based study from British Columbia.Br J Haematol. 2014 Dec;167(5):608-17. doi: 10.1111/bjh.13085. Epub 2014 Aug 19. Br J Haematol. 2014. PMID: 25135752
-
MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program.Blood. 2013 May 16;121(20):4021-31; quiz 4250. doi: 10.1182/blood-2012-10-460063. Epub 2013 Feb 28. Blood. 2013. PMID: 23449635 Free PMC article.
-
How gene polymorphisms can influence clinical response and toxicity following R-CHOP therapy in patients with diffuse large B cell lymphoma.Blood Rev. 2017 Jul;31(4):235-249. doi: 10.1016/j.blre.2017.02.005. Epub 2017 Feb 27. Blood Rev. 2017. PMID: 28262268 Review.
-
Beyond RCHOP: A Blueprint for Diffuse Large B Cell Lymphoma Research.J Natl Cancer Inst. 2016 Dec 16;108(12):djw257. doi: 10.1093/jnci/djw257. Print 2016 Dec. J Natl Cancer Inst. 2016. PMID: 27986884 Free PMC article. Review.
Cited by
-
Primary splenic diffuse large B-cell lymphoma presenting as a splenic abscess.EJHaem. 2023 Jan 24;4(1):226-231. doi: 10.1002/jha2.642. eCollection 2023 Feb. EJHaem. 2023. PMID: 36819150 Free PMC article.
-
Spanish Lymphoma Group (GELTAMO) guidelines for the diagnosis, staging, treatment, and follow-up of diffuse large B-cell lymphoma.Oncotarget. 2018 Aug 17;9(64):32383-32399. doi: 10.18632/oncotarget.25892. eCollection 2018 Aug 17. Oncotarget. 2018. PMID: 30190794 Free PMC article. Review.
-
Brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone as frontline treatment for patients with CD30-positive B-cell lymphomas.Haematologica. 2021 Jun 1;106(6):1705-1713. doi: 10.3324/haematol.2019.238675. Haematologica. 2021. PMID: 32414850 Free PMC article. Clinical Trial.
-
Epstein-Barr virus positive diffuse large B-cell lymphoma predict poor outcome, regardless of the age.Sci Rep. 2015 Jul 23;5:12168. doi: 10.1038/srep12168. Sci Rep. 2015. PMID: 26202875 Free PMC article.
-
Novel Targets and Advanced Therapies in Diffuse Large B Cell Lymphomas.Cancers (Basel). 2024 Jun 17;16(12):2243. doi: 10.3390/cancers16122243. Cancers (Basel). 2024. PMID: 38927948 Free PMC article. Review.
References
-
- Stein H, Warnke RA, Chan WC, et al. Diffuse large B-cell lymphoma, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2008. pp. 233–237.
-
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. - PubMed
-
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. - PubMed
-
- Muris JJ, Meijer CJ, Vos W, et al. Immunohistochemical profiling based on Bcl-2, CD10 and MUM1 expression improves risk stratification in patients with primary nodal diffuse large B cell lymphoma. J Pathol. 2006;208(5):714–723. - PubMed
-
- Nyman H, Adde M, Karjalainen-Lindsberg ML, et al. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood. 2007;109(11):4930–4935. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

