Immobile survival of motoneuron (SMN) protein stored in Cajal bodies can be mobilized by protein interactions
- PMID: 23334184
- PMCID: PMC11113639
- DOI: 10.1007/s00018-012-1242-8
Immobile survival of motoneuron (SMN) protein stored in Cajal bodies can be mobilized by protein interactions
Abstract
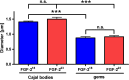
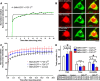
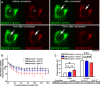
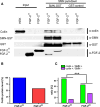
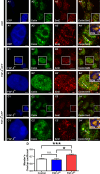
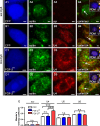
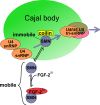
Reduced levels of survival of motoneuron (SMN) protein lead to spinal muscular atrophy, but it is still unknown how SMN protects motoneurons in the spinal cord against degeneration. In the nucleus, SMN is associated with two types of nuclear bodies denoted as gems and Cajal bodies (CBs). The 23 kDa isoform of fibroblast growth factor-2 (FGF-2(23)) is a nuclear protein that binds to SMN and destabilizes the SMN-Gemin2 complex. In the present study, we show that FGF-2(23) depletes SMN from CBs without affecting their general structure. FRAP analysis of SMN-EGFP in CBs demonstrated that the majority of SMN in CBs remained mobile and allowed quantification of fast, slow and immobile nuclear SMN populations. The potential for SMN release was confirmed by in vivo photoconversion of SMN-Dendra2, indicating that CBs concentrate immobile SMN that could have a specialized function in CBs. FGF-2(23) accelerated SMN release from CBs, accompanied by a conversion of immobile SMN into a mobile population. Furthermore, FGF-2(23) caused snRNP accumulation in CBs. We propose a model in which Cajal bodies store immobile SMN that can be mobilized by its nuclear interaction partner FGF-2(23), leading to U4 snRNP accumulation in CBs, indicating a role for immobile SMN in tri-snRNP assembly.
Figures

Similar articles
-
Regulation of neuronal differentiation by proteins associated with nuclear bodies.PLoS One. 2013 Dec 17;8(12):e82871. doi: 10.1371/journal.pone.0082871. eCollection 2013. PLoS One. 2013. PMID: 24358231 Free PMC article.
-
Nuclear gems and Cajal (coiled) bodies in fetal tissues: nucleolar distribution of the spinal muscular atrophy protein, SMN.Exp Cell Res. 2001 May 1;265(2):252-61. doi: 10.1006/excr.2001.5186. Exp Cell Res. 2001. PMID: 11302690
-
Fibroblast growth factor-2 regulates the stability of nuclear bodies.Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):12747-52. doi: 10.1073/pnas.0900122106. Epub 2009 Jul 17. Proc Natl Acad Sci U S A. 2009. PMID: 19617559 Free PMC article.
-
SMN - A chaperone for nuclear RNP social occasions?RNA Biol. 2017 Jun 3;14(6):701-711. doi: 10.1080/15476286.2016.1236168. Epub 2016 Sep 20. RNA Biol. 2017. PMID: 27648855 Free PMC article. Review.
-
The Cajal body.Biochim Biophys Acta. 2008 Nov;1783(11):2108-15. doi: 10.1016/j.bbamcr.2008.07.016. Epub 2008 Aug 3. Biochim Biophys Acta. 2008. PMID: 18755223 Review.
Cited by
-
A nuclear odyssey: fibroblast growth factor-2 (FGF-2) as a regulator of nuclear homeostasis in the nervous system.Cell Mol Life Sci. 2015 May;72(9):1651-62. doi: 10.1007/s00018-014-1818-6. Epub 2015 Jan 1. Cell Mol Life Sci. 2015. PMID: 25552245 Free PMC article. Review.
-
Regulation of neuronal differentiation by proteins associated with nuclear bodies.PLoS One. 2013 Dec 17;8(12):e82871. doi: 10.1371/journal.pone.0082871. eCollection 2013. PLoS One. 2013. PMID: 24358231 Free PMC article.
-
What could be the function of the spinal muscular atrophy-causing protein SMN in macrophages?Front Immunol. 2024 May 28;15:1375428. doi: 10.3389/fimmu.2024.1375428. eCollection 2024. Front Immunol. 2024. PMID: 38863697 Free PMC article. Review.
-
Signals controlling Cajal body assembly and function.Int J Biochem Cell Biol. 2013 Jul;45(7):1314-7. doi: 10.1016/j.biocel.2013.03.019. Epub 2013 Apr 10. Int J Biochem Cell Biol. 2013. PMID: 23583661 Free PMC article. Review.
-
A Single Amino Acid Residue Regulates PTEN-Binding and Stability of the Spinal Muscular Atrophy Protein SMN.Cells. 2020 Nov 3;9(11):2405. doi: 10.3390/cells9112405. Cells. 2020. PMID: 33153033 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

