Peptidomics methods for the identification of peptidase-substrate interactions
- PMID: 23332665
- PMCID: PMC3594040
- DOI: 10.1016/j.cbpa.2012.10.038
Peptidomics methods for the identification of peptidase-substrate interactions
Abstract
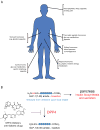
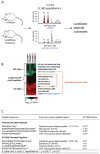
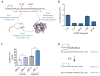
Peptidases have important roles in controlling physiological signaling through their regulation of bioactive peptides. Understanding and controlling bioactive peptide regulation is of great biomedical interest and approaches that elucidate the interplay between peptidases and their substrates are vital for achieving this goal. Here, we highlight the utility of recent peptidomics approaches in identifying endogenous substrates of peptidases. These approaches reveal bioactive substrates and help characterize the biochemical functions of the enzyme. Most recently, peptidomics approaches have been applied to address the challenging question of identifying the peptidases responsible for regulating specific bioactive peptides. Since peptidases are of great biomedical interest, these approaches will begin to impact our ability to identify new drug targets that regulate important bioactive peptides.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Peptidomics approach to elucidate the proteolytic regulation of bioactive peptides.Proc Natl Acad Sci U S A. 2012 May 29;109(22):8523-7. doi: 10.1073/pnas.1203195109. Epub 2012 May 14. Proc Natl Acad Sci U S A. 2012. PMID: 22586115 Free PMC article.
-
Analysis of the proteolysis of bioactive peptides using a peptidomics approach.Nat Protoc. 2013 Sep;8(9):1730-42. doi: 10.1038/nprot.2013.104. Epub 2013 Aug 15. Nat Protoc. 2013. PMID: 23949379 Free PMC article.
-
Expanding the dipeptidyl peptidase 4-regulated peptidome via an optimized peptidomics platform.J Am Chem Soc. 2010 Mar 24;132(11):3819-30. doi: 10.1021/ja909524e. J Am Chem Soc. 2010. PMID: 20178363 Free PMC article.
-
Peptidomics of the prolyl peptidases.AAPS J. 2010 Dec;12(4):483-91. doi: 10.1208/s12248-010-9208-y. Epub 2010 Jun 16. AAPS J. 2010. PMID: 20552307 Free PMC article. Review.
-
Investigating endogenous peptides and peptidases using peptidomics.Biochemistry. 2011 Sep 6;50(35):7447-61. doi: 10.1021/bi200417k. Epub 2011 Aug 15. Biochemistry. 2011. PMID: 21786763 Free PMC article. Review.
Cited by
-
An Efficient Peptidomics Screening for Exogenous Substrates and Inhibitory Peptides of the Dipeptidase ACE from Milk Hydrolysate.Pharmaceutics. 2023 Jan 27;15(2):425. doi: 10.3390/pharmaceutics15020425. Pharmaceutics. 2023. PMID: 36839747 Free PMC article.
-
[A differential peptidomics analysis of hippocampal tissue in a rat model of premature white matter injury].Zhongguo Dang Dai Er Ke Za Zhi. 2019 Nov;21(11):1116-1123. doi: 10.7499/j.issn.1008-8830.2019.11.012. Zhongguo Dang Dai Er Ke Za Zhi. 2019. PMID: 31753095 Free PMC article. Chinese.
-
Peptidomics Analysis Discloses That Novel Bioactive Peptides Participate in Necrotizing Enterocolitis in a Rat Model.Biomed Res Int. 2020 Dec 31;2020:4705149. doi: 10.1155/2020/4705149. eCollection 2020. Biomed Res Int. 2020. PMID: 33490244 Free PMC article.
-
Profiling Analysis Reveals the Crucial Role of the Endogenous Peptides in Bladder Cancer Progression.Onco Targets Ther. 2020 Dec 3;13:12443-12455. doi: 10.2147/OTT.S281713. eCollection 2020. Onco Targets Ther. 2020. PMID: 33311987 Free PMC article.
-
Peptidomics for the discovery and characterization of neuropeptides and hormones.Trends Pharmacol Sci. 2015 Sep;36(9):579-86. doi: 10.1016/j.tips.2015.05.009. Epub 2015 Jul 1. Trends Pharmacol Sci. 2015. PMID: 26143240 Free PMC article. Review.
References
-
- De Felipe C, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

