Molecular control of steady-state dendritic cell maturation and immune homeostasis
- PMID: 23330953
- PMCID: PMC4091962
- DOI: 10.1146/annurev-immunol-020711-074929
Molecular control of steady-state dendritic cell maturation and immune homeostasis
Abstract
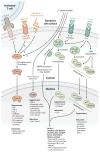
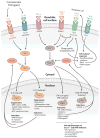
Dendritic cells (DCs) are specialized sentinels responsible for coordinating adaptive immunity. This function is dependent upon coupled sensitivity to environmental signs of inflammation and infection to cellular maturation-the programmed alteration of DC phenotype and function to enhance immune cell activation. Although DCs are thus well equipped to respond to pathogens, maturation triggers are not unique to infection. Given that immune cells are exquisitely sensitive to the biological functions of DCs, we now appreciate that multiple layers of suppression are required to restrict the environmental sensitivity, cellular maturation, and even life span of DCs to prevent aberrant immune activation during the steady state. At the same time, steady-state DCs are not quiescent but rather perform key functions that support homeostasis of numerous cell types. Here we review these functions and molecular mechanisms of suppression that control steady-state DC maturation. Corruption of these steady-state operatives has diverse immunological consequences and pinpoints DCs as potent drivers of autoimmune and inflammatory disease.
Figures
Similar articles
-
TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses.J Immunol. 2004 Dec 1;173(11):6882-9. doi: 10.4049/jimmunol.173.11.6882. J Immunol. 2004. PMID: 15557183
-
Endotoxin-induced maturation of MyD88-deficient dendritic cells.J Immunol. 2001 May 1;166(9):5688-94. doi: 10.4049/jimmunol.166.9.5688. J Immunol. 2001. PMID: 11313410
-
Self- and nonself-recognition by C-type lectins on dendritic cells.Annu Rev Immunol. 2004;22:33-54. doi: 10.1146/annurev.immunol.22.012703.104558. Annu Rev Immunol. 2004. PMID: 15032573 Review.
-
IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation.J Immunol. 2004 Sep 15;173(6):3844-54. doi: 10.4049/jimmunol.173.6.3844. J Immunol. 2004. PMID: 15356132
-
Regulation of the tolerogenic function of steady-state DCs.Eur J Immunol. 2014 Apr;44(4):927-33. doi: 10.1002/eji.201343862. Epub 2014 Mar 20. Eur J Immunol. 2014. PMID: 24652744 Review.
Cited by
-
Manipulating antigen presentation for antigen-specific immunotherapy of autoimmune diseases.Curr Opin Immunol. 2021 Jun;70:75-81. doi: 10.1016/j.coi.2021.03.019. Epub 2021 Apr 18. Curr Opin Immunol. 2021. PMID: 33878516 Free PMC article. Review.
-
Elucidating the Role of Ezh2 in Tolerogenic Function of NOD Bone Marrow-Derived Dendritic Cells Expressing Constitutively Active Stat5b.Int J Mol Sci. 2020 Sep 4;21(18):6453. doi: 10.3390/ijms21186453. Int J Mol Sci. 2020. PMID: 32899608 Free PMC article.
-
Induction of antitumor cytotoxic lymphocytes using engineered human primary blood dendritic cells.Proc Natl Acad Sci U S A. 2018 May 8;115(19):E4453-E4462. doi: 10.1073/pnas.1800550115. Epub 2018 Apr 19. Proc Natl Acad Sci U S A. 2018. PMID: 29674449 Free PMC article.
-
The non-canonical NF-κB pathway in immunity and inflammation.Nat Rev Immunol. 2017 Sep;17(9):545-558. doi: 10.1038/nri.2017.52. Epub 2017 Jun 5. Nat Rev Immunol. 2017. PMID: 28580957 Free PMC article. Review.
-
Roles of Ubiquitination and Deubiquitination in Regulating Dendritic Cell Maturation and Function.Front Immunol. 2020 Nov 16;11:586613. doi: 10.3389/fimmu.2020.586613. eCollection 2020. Front Immunol. 2020. PMID: 33329564 Free PMC article. Review.
References
-
- Nobel Assem. Karolinska Inst. The gatekeepers of the immune system. 2011 Nobel Prize in Physiology or Medicine: Popular Information. 2011:8. http://www.nobelprize.org/nobel_prizes/medicine/laureates/2011/popular-m....
-
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. - PubMed
-
- Banchereau J, Brière F, Caux C, Davoust J, Lebecque S, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
-
- Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

