p120-catenin and β-catenin differentially regulate cadherin adhesive function
- PMID: 23325790
- PMCID: PMC3596243
- DOI: 10.1091/mbc.E12-06-0471
p120-catenin and β-catenin differentially regulate cadherin adhesive function
Abstract
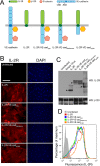
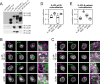
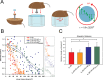
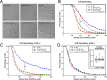
Vascular endothelial (VE)-cadherin, the major adherens junction adhesion molecule in endothelial cells, interacts with p120-catenin and β-catenin through its cytoplasmic tail. However, the specific functional contributions of the catenins to the establishment of strong adhesion are not fully understood. Here we use bioengineering approaches to identify the roles of cadherin-catenin interactions in promoting strong cellular adhesion and the ability of the cells to spread on an adhesive surface. Our results demonstrate that the domain of VE-cadherin that binds to β-catenin is required for the establishment of strong steady-state adhesion strength. Surprisingly, p120 binding to the cadherin tail had no effect on the strength of adhesion when the available adhesive area was limited. Instead, the binding of VE-cadherin to p120 regulates adhesive contact area in a Rac1-dependent manner. These findings reveal that p120 and β-catenin have distinct but complementary roles in strengthening cadherin-mediated adhesion.
Figures


Similar articles
-
Phosphorylation of VE-cadherin controls endothelial phenotypes via p120-catenin coupling and Rac1 activation.Am J Physiol Heart Circ Physiol. 2011 Jan;300(1):H162-72. doi: 10.1152/ajpheart.00650.2010. Epub 2010 Oct 29. Am J Physiol Heart Circ Physiol. 2011. PMID: 21037229 Free PMC article.
-
Distribution of adherens junction mediated by VE-cadherin complex in rat spleen sinus endothelial cells.Cell Tissue Res. 2006 Mar;323(3):417-24. doi: 10.1007/s00441-005-0064-5. Epub 2005 Oct 22. Cell Tissue Res. 2006. PMID: 16244888
-
p120-catenin binding masks an endocytic signal conserved in classical cadherins.J Cell Biol. 2012 Oct 15;199(2):365-80. doi: 10.1083/jcb.201205029. J Cell Biol. 2012. PMID: 23071156 Free PMC article.
-
Protecting your tail: regulation of cadherin degradation by p120-catenin.Curr Opin Cell Biol. 2004 Oct;16(5):522-7. doi: 10.1016/j.ceb.2004.07.001. Curr Opin Cell Biol. 2004. PMID: 15363802 Review.
-
p120-Catenin: a novel regulator of innate immunity and inflammation.Crit Rev Immunol. 2012;32(2):127-38. doi: 10.1615/critrevimmunol.v32.i2.20. Crit Rev Immunol. 2012. PMID: 23216611 Free PMC article. Review.
Cited by
-
A novel noncanonical function for IRF6 in the recycling of E-cadherin.Mol Biol Cell. 2024 Jul 1;35(7):ar102. doi: 10.1091/mbc.E23-11-0430. Epub 2024 May 29. Mol Biol Cell. 2024. PMID: 38809584 Free PMC article.
-
p120-catenin regulates VE-cadherin endocytosis and degradation induced by the Kaposi sarcoma-associated ubiquitin ligase K5.Mol Biol Cell. 2017 Jan 1;28(1):30-40. doi: 10.1091/mbc.E16-06-0459. Epub 2016 Oct 26. Mol Biol Cell. 2017. PMID: 27798235 Free PMC article.
-
Phosphoregulation of the C. elegans cadherin-catenin complex.Biochem J. 2015 Dec 15;472(3):339-52. doi: 10.1042/BJ20150410. Epub 2015 Oct 6. Biochem J. 2015. PMID: 26443865 Free PMC article.
-
Ginsenoside Rg1 Improves Differentiation by Inhibiting Senescence of Human Bone Marrow Mesenchymal Stem Cell via GSK-3β and β-Catenin.Stem Cells Int. 2020 May 26;2020:2365814. doi: 10.1155/2020/2365814. eCollection 2020. Stem Cells Int. 2020. PMID: 32565825 Free PMC article.
-
A composite model of the human postcapillary venule for investigation of microvascular leukocyte recruitment.FASEB J. 2014 Mar;28(3):1166-80. doi: 10.1096/fj.13-240986. Epub 2013 Dec 2. FASEB J. 2014. PMID: 24297702 Free PMC article.
References
-
- Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. - PubMed
-
- Anastasiadis PZ. p120-ctn: a nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta. 2007;1773:34–46. - PubMed
-
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2:637–644. - PubMed
-
- Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010;103:40–55. - PubMed
-
- Bodeau AL, Berrier AL, Mastrangelo AM, Martinez R, LaFlamme SE. A functional comparison of mutations in integrin beta cytoplasmic domains: effects on the regulation of tyrosine phosphorylation, cell spreading, cell attachment and beta1 integrin conformation. J Cell Sci. 2001;114:2795–2807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AR050501/AR/NIAMS NIH HHS/United States
- F30 HL110447/HL/NHLBI NIH HHS/United States
- T32 GM008169/GM/NIGMS NIH HHS/United States
- F30HL110447/HL/NHLBI NIH HHS/United States
- R01HL77870/HL/NHLBI NIH HHS/United States
- T32 EY007092/EY/NEI NIH HHS/United States
- T32 GM008490/GM/NIGMS NIH HHS/United States
- R01 HL077870/HL/NHLBI NIH HHS/United States
- T32EY007092/EY/NEI NIH HHS/United States
- T32 GM008367/GM/NIGMS NIH HHS/United States
- R01 AR050501/AR/NIAMS NIH HHS/United States
- R01 GM065918/GM/NIGMS NIH HHS/United States
- R01GM065918/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

