Remanent cell traction force in renal vascular smooth muscle cells induced by integrin-mediated mechanotransduction
- PMID: 23325413
- PMCID: PMC3566537
- DOI: 10.1152/ajpcell.00234.2012
Remanent cell traction force in renal vascular smooth muscle cells induced by integrin-mediated mechanotransduction
Abstract
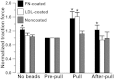
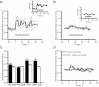
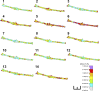
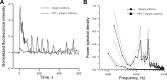
It was previously demonstrated in isolated renal vascular smooth muscle cells (VSMCs) that integrin-mediated mechanotransduction triggers intracellular Ca(2+) mobilization, which is the hallmark of myogenic response in VSMCs. To test directly whether integrin-mediated mechanotransduction results in the myogenic response-like behavior in renal VSMCs, cell traction force microscopy was used to monitor cell traction force when the cells were pulled with fibronectin-coated or low density lipoprotein (LDL)-coated paramagnetic beads. LDL-coated beads were used as a control for nonintegrin-mediated mechanotransduction. Pulling with LDL-coated beads increased the cell traction force by 61 ± 12% (9 cells), which returned to the prepull level after the pulling process was terminated. Pulling with noncoated beads had a minimal increase in the cell traction force (12 ± 9%, 8 cells). Pulling with fibronectin-coated beads increased the cell traction force by 56 ± 20% (7 cells). However, the cell traction force was still elevated by 23 ± 14% after the pulling process was terminated. This behavior is analogous to the changes of vascular resistance in pressure-induced myogenic response, in which vascular resistance remains elevated after myogenic constriction. Fibronectin is a native ligand for α(5)β(1)-integrins in VSMCs. Similar remanent cell traction force was found when cells were pulled with beads coated with β(1)-integrin antibody (Ha2/5). Activation of β(1)-integrin with soluble antibody also triggered variations of cell traction force and Ca(2+) mobilization, which were abolished by the Src inhibitor. In conclusion, mechanical force transduced by α(5)β(1)-integrins triggered a myogenic response-like behavior in isolated renal VSMCs.
Figures


Similar articles
-
Integrin-mediated mechanotransduction in renal vascular smooth muscle cells: activation of calcium sparks.Am J Physiol Regul Integr Comp Physiol. 2007 Oct;293(4):R1586-94. doi: 10.1152/ajpregu.00025.2007. Epub 2007 Aug 15. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 17699564
-
Mechanotransduction through fibronectin-integrin focal adhesion in microvascular smooth muscle cells: is calcium essential?Am J Physiol Heart Circ Physiol. 2012 May 15;302(10):H1965-73. doi: 10.1152/ajpheart.00598.2011. Epub 2012 Mar 16. Am J Physiol Heart Circ Physiol. 2012. PMID: 22427509 Free PMC article.
-
Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites.Am J Physiol Cell Physiol. 2008 Jul;295(1):C268-78. doi: 10.1152/ajpcell.00516.2007. Epub 2008 May 21. Am J Physiol Cell Physiol. 2008. PMID: 18495809 Free PMC article.
-
Coordinated regulation of vascular Ca2+ and K+ channels by integrin signaling.Adv Exp Med Biol. 2010;674:69-79. doi: 10.1007/978-1-4419-6066-5_7. Adv Exp Med Biol. 2010. PMID: 20549941 Review.
-
Arteriolar vascular smooth muscle cells: mechanotransducers in a complex environment.Int J Biochem Cell Biol. 2012 Sep;44(9):1505-10. doi: 10.1016/j.biocel.2012.05.021. Epub 2012 Jun 5. Int J Biochem Cell Biol. 2012. PMID: 22677491 Free PMC article. Review.
Cited by
-
Contractile dynamics change before morphological cues during fluorescence [corrected] illumination.Sci Rep. 2015 Dec 22;5:18513. doi: 10.1038/srep18513. Sci Rep. 2015. PMID: 26691776 Free PMC article.
-
Upregulated desmin/integrin β1/MAPK axis promotes elastic cartilage regeneration with increased ECM mechanical strength.Int J Biol Sci. 2023 May 21;19(9):2740-2755. doi: 10.7150/ijbs.83024. eCollection 2023. Int J Biol Sci. 2023. PMID: 37324935 Free PMC article.
-
Reduced stiffness and augmented traction force in type 2 diabetic coronary microvascular smooth muscle.Am J Physiol Heart Circ Physiol. 2020 Jun 1;318(6):H1410-H1419. doi: 10.1152/ajpheart.00542.2019. Epub 2020 May 1. Am J Physiol Heart Circ Physiol. 2020. PMID: 32357115 Free PMC article.
-
Guidelines for the isolation and characterization of murine vascular smooth muscle cells. A report from the International Society of Cardiovascular Translational Research.J Cardiovasc Transl Res. 2015 Apr;8(3):158-63. doi: 10.1007/s12265-015-9616-6. Epub 2015 Mar 19. J Cardiovasc Transl Res. 2015. PMID: 25788147 Free PMC article.
-
Intraluminal pressure triggers myogenic response via activation of calcium spark and calcium-activated chloride channel in rat renal afferent arteriole.Am J Physiol Renal Physiol. 2018 Dec 1;315(6):F1592-F1600. doi: 10.1152/ajprenal.00239.2018. Epub 2018 Aug 8. Am J Physiol Renal Physiol. 2018. PMID: 30089032 Free PMC article.
References
-
- Balasubramanian L, Ahmed A, Lo CM, Sham JS, Yip KP. Integrin-mediated mechanotransduction in renal vascular smooth muscle cells: activation of calcium sparks. Am J Physiol Regul Integr Comp Physiol 293: R1586–R1594, 2007 - PubMed
-
- Beningo KA, Lo CM, Wang YL. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol 69: 325–339, 2002 - PubMed
-
- Carmines PK, Inscho EW, Gensure RC. Arterial pressure effects on preglomerular microvasculature of juxtamedullary nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 258: F94–F102, 1990 - PubMed
-
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262: 740–744, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

